Why does #"HF"# have a lower boiling point than water even though #"F"# is more electronegative than #"O"#?
1 Answer
There are many complicated factors. This is what I could find:
- Water can make a more-balanced hydrogen-bonding network (two acceptors and two donors, instead of one donor and three acceptors), making the bulk system more uniformly interacting and thus stronger as a whole.
- Water forms a more extensive hydrogen-bonding network (a three-dimensional tetrahedral local environment, rather than a two-dimensional zigzag environment), rendering the bulk system less easily vaporizable.
- Water generally has a more optimal angle of interaction, as it more closely matches the electron geometry of the molecule and aligns the interactions along the molecular dipole, whereas
#"HF"# would have stronger hydrogen-bonding (in terms of raw numbers) if the interactions were linear.
DISCLAIMER: LONG ANSWER! Also very visual.
Hydrogen-bonding is the strongest intermolecular force in both
When we draw out all the hydrogen-bonding interactions, and assume pure water and pure
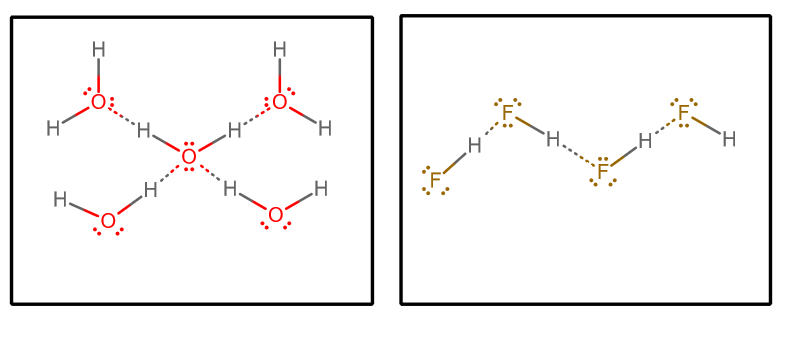
We could then consider a few factors:
- Number of hydrogen-bonding donors and acceptors
Each water molecule can accept two hydrogen-bonding interactions (via the lone pairs) and donate two hydrogen-bonding interactions (via the hydrogens).
On the other hand, each
#"HF"# molecule accepts three hydrogen-bonding interactions (via the lone pairs) and donates only one (via the hydrogen).One could argue then that water has a more balanced hydrogen-bonding network, which renders the bulk system less able to vaporize overall. This would support the higher boiling point of water over
#"HF"# .
- Electronegativities of
#bb("F")# and#bb("O")#
#"F"# is more electronegative, so it holds onto its electron density more easily; thus, the lone pairs are expected to be weaker donors of hydrogen-bonding interactions than the lone pairs on#"O"# .This would seem to predict that each individual hydrogen-bonding interaction is weaker, which would support the higher boiling point of water over
#"HF"# , BUT this is contradicted by the following point.
- Average raw hydrogen-bonding strengths in
#bb("HF")# vs. water
#"O"-"H"cdotcdotcdot:"O"-# ,#DeltaH_("H"-"bond") ~~ "21 kJ/mol"#
#"F"-"H"cdotcdotcdot:"F"-# ,#DeltaH_("H"-"bond") ~~ "161.5 kJ/mol"# This data from Wikipedia suggests that
#"HF"# has stronger hydrogen-bonding interactions amongst#"HF"# molecules than in water.This would not support the experimental proof that
#"HF"# has a much lower boiling point, but this also is just a raw number and does not consider, say, interaction angles in the bulk system.
- Observed hydrogen-bonding angles and dimensions of interaction
#"HF"# , being a linear molecule, has been seen to have zigzag hydrogen-bonding interactions, in two dimensions, with angles of#116^@# . A more optimal angle would be at#180^@# , since that would give more direct dipole interactions. This limits the hydrogen-bonding strength of#"HF"# .
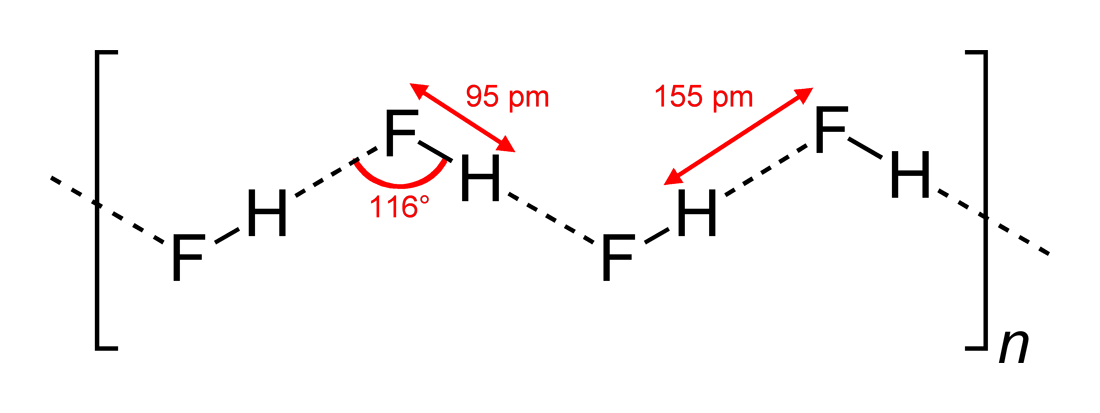
Water, being a bent molecule with a tetrahedral electron geometry, can hydrogen-bond in three dimensions.
Here's an example of the tetrahedral cluster found in the local environment of a hydrogen-bonding interaction in water:
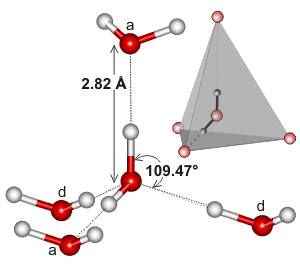
It may be that the extensivity of the hydrogen-bonding network, and how it tends to match the electron geometry of water, is what makes water's boiling point so high. This would support the higher boiling point of water over
#"HF"# .
Overall, these are the least-challenged factors I could think of:
- Water can make a more-balanced hydrogen-bonding network (two acceptors and two donors, instead of one donor and three acceptors), making the bulk system more uniformly interacting and thus stronger as a whole.
- Water forms a more extensive hydrogen-bonding network (a three-dimensional tetrahedral local environment, rather than a two-dimensional zigzag environment), rendering the bulk system less easily vaporizable.
- Water generally has a more optimal angle of interaction, as it more closely matches the electron geometry of the molecule and aligns the interactions along the molecular dipole, whereas
#"HF"# would have stronger hydrogen-bonding (in terms of raw numbers) if the interactions were linear.

