Which type of bond is formed when Ammonia and hydrogen ion bond together to form ammonium ion?
2 Answers
Dative covalent bond/coordinate bond.
Explanation:
When one atom supplies both electrons in forming a two-electron bond, it is known as a dative covalent or coordinate bond. If two atoms contribute one electron each, it is known as a simple covalent bond.
In this case, the nitrogen atom has a lone pair of electrons that coordinates to the hydrogen cation, which has zero electrons, forming a covalent bond. The resultant bond is now indistinguishable from the other three nitrogen-hydrogen bonds. This is important. The difference between simple and dative covalent bonds is in how they are made, but after they are made, they are the same bond.
For example you can hypothetically form hydrogen in two ways, giving the same molecule
Dative covalent
Simple covalent
Covalent bonds are formed. More specifically,
Explanation:
Covalent bonds are formed when electrons are shared between elements that are nonmetals.
The ammonium ion,
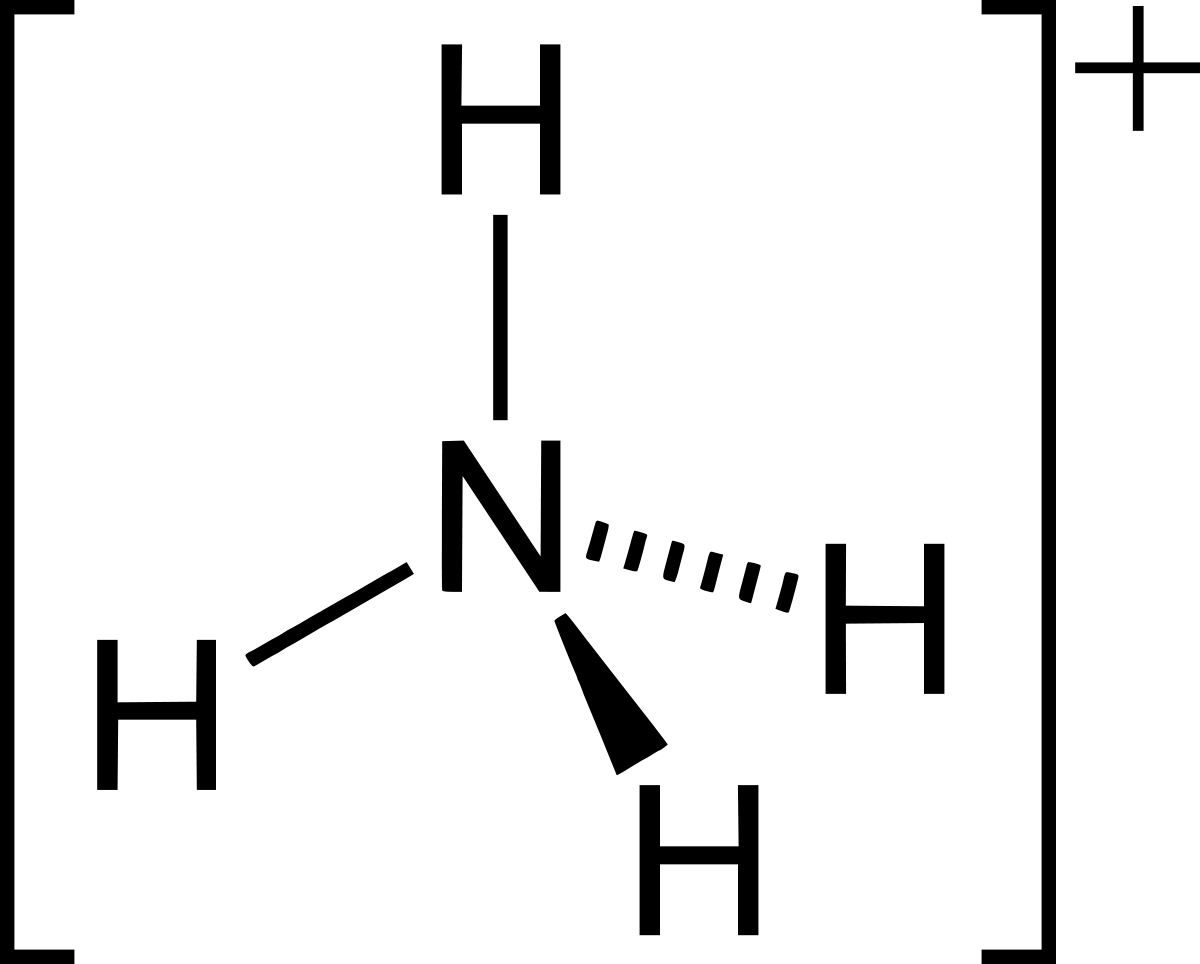 Wikipedia
Wikipedia
Of these covalent bonds,
This is because, when the
one hydrogen ion is transferred from
Chemguide
So, the bond between this particular hydrogen atom and the central nitrogen is a dative covalent bond.
The rest of the bonds all contain electrons from both hydrogen and nitrogen, so they would be considered ordinary covalent bonds.


