Are reduction reactions always endothermic?
1 Answer
On the contrary, they are usually exothermic...
...as can be seen in a table of electron affinities:
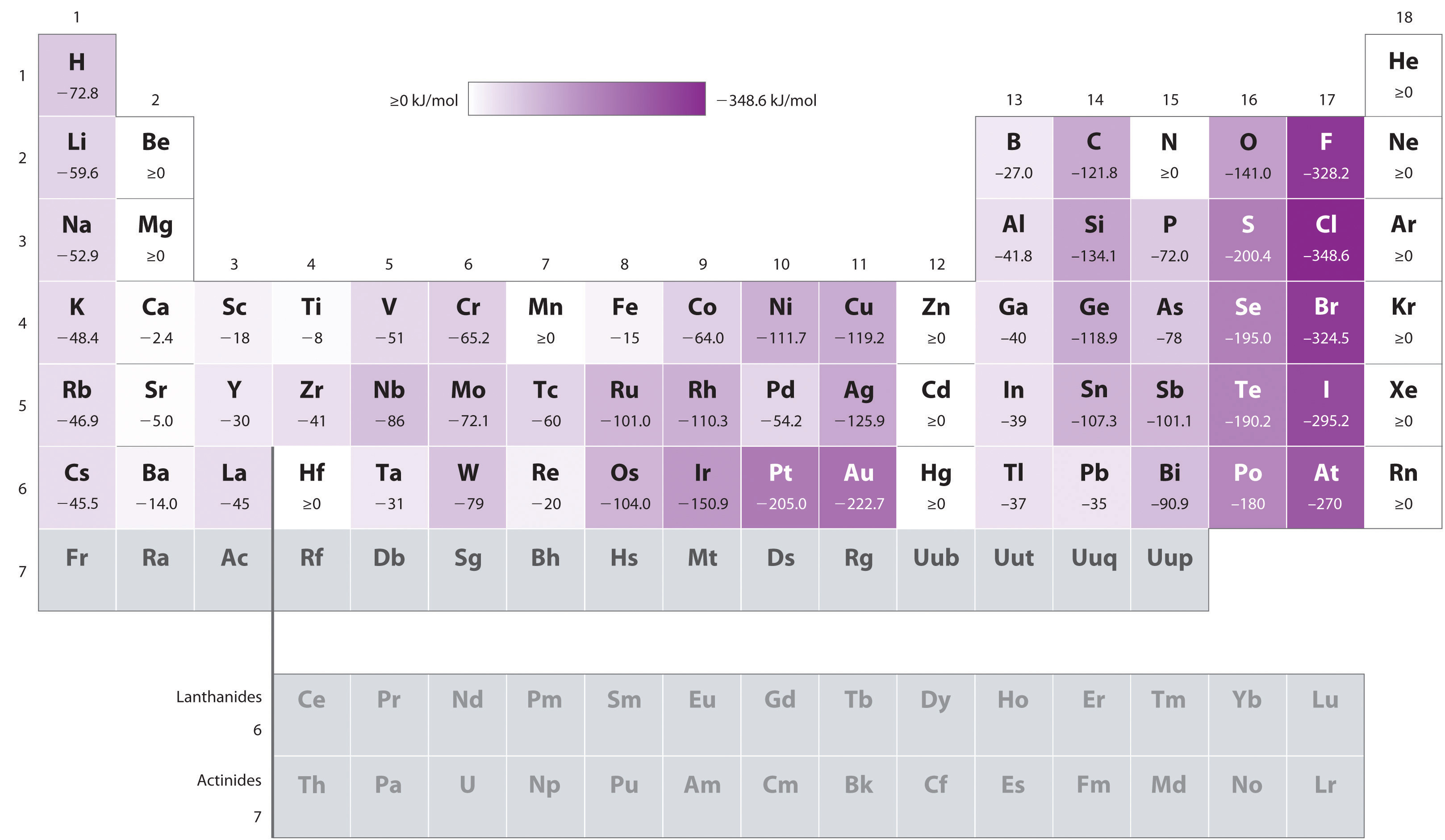
...which are given as:
#"M"(g) + ze^(-) -> "M"^(z-)(g)# #" "bb((1))#
or
#"M"^(n-)(g) + ze^(-) ->"M"^((n+z)-)(g)# #" "bb((2))# where
#n-# is an integer negative charge, and#z# is the number of electrons added into the atom.
Case
We take case
- Does the new electron go into a new, higher energy level? If so, we expect the process to be endothermic.
This is typical of noble gases and alkaline earth metals.
Some examples are beryllium and magnesium, as well as all the noble gases.
[However, calcium, strontium, and barium have slightly exothermic reduction processes, since the upper energy levels are closer together, making say, barium, have a more exothermic electron affinity than strontium.]
- Does the new electron cause primarily more electron repulsion within the same energy level? If so, we expect the process to be endothermic.
As for examples, this is only known for the neutral nitrogen and manganese atoms, apparently.
- Does the new electron stabilize the atom? If so, we expect the process to be exothermic.
This is typical of the alkali metal and halogen groups.
Examples? All of their electron affinities are exothermic.

