Define dispersion force? How does this dispersion force result in an attractive intermolecular force?
So I need to define the following terms and give an example. The term I need to do was Dispersion Force.
My answer: The London dispersion force is a temporary attractive force that happens when the electrons occupy positions that make the atoms form temporary dipoles. An example of this is often found in the halogens, noble gases, an in other non-polar molecules.
My teacher wants me to also answer, how does this dispersion force result in an attractive intermolecular force?
Can someone help me with this?
So I need to define the following terms and give an example. The term I need to do was Dispersion Force.
My answer: The London dispersion force is a temporary attractive force that happens when the electrons occupy positions that make the atoms form temporary dipoles. An example of this is often found in the halogens, noble gases, an in other non-polar molecules.
My teacher wants me to also answer, how does this dispersion force result in an attractive intermolecular force?
Can someone help me with this?
1 Answer
Consider the temporary attractive forces you describe.
When electrons are at positions conducive to producing transient dipoles with nearby nuclei of other molecules, there are intermolecular forces at play.
Assume the black dot to be where the electron density is, where there is a partial negative charge, and the grey regions where there is no electron density to be where the partial positive charge is.
Taking into consideration the preceding, apply Coloumb's law: negative charges attract positive charges. Hence,
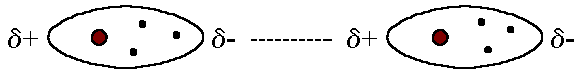
In a biological system, these very forces contribute to the hydrophobic effect which promotes protein folding and phospholipid membrane formation, among other things.

