What is the standard state free energy for this reaction?
Gold ions form complexes with cyanide ion according to the equation:
Au+(aq) + 2 CN <-> Au(CN)2 kf=2x10^38
What is the standard state free energy for this reaction?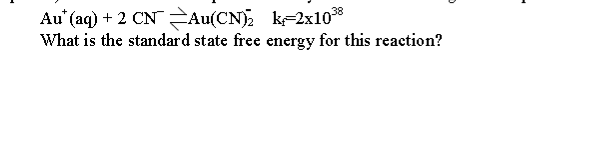
Gold ions form complexes with cyanide ion according to the equation:
Au+(aq) + 2 CN <-> Au(CN)2 kf=2x10^38
What is the standard state free energy for this reaction?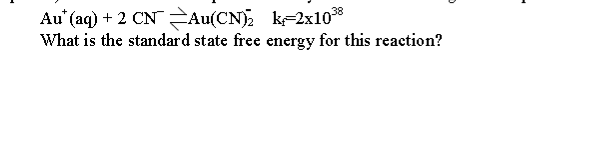
1 Answer
Explanation:
The equation
#K_"eq" = e^(-(Delta G^ "o")/("R" cdot "T"))#
Relates the equilibrium constant of a reaction,
Solve for

