Do metals have low ionization energies?
1 Answer
Jul 6, 2018
In general, they do...
Explanation:
Metals are typically REDUCING materials, and the data reflect this. And metals come from the left hand side of the Periodic Table as we face it...with LARGER atomic radii, so that the nuclear charge, i.e.
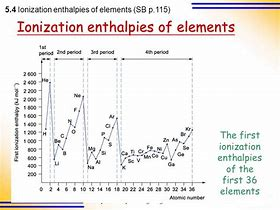
And so metals, TEND to have LOW ionization enthalpies in comparison to the non-metals of the same Period....

