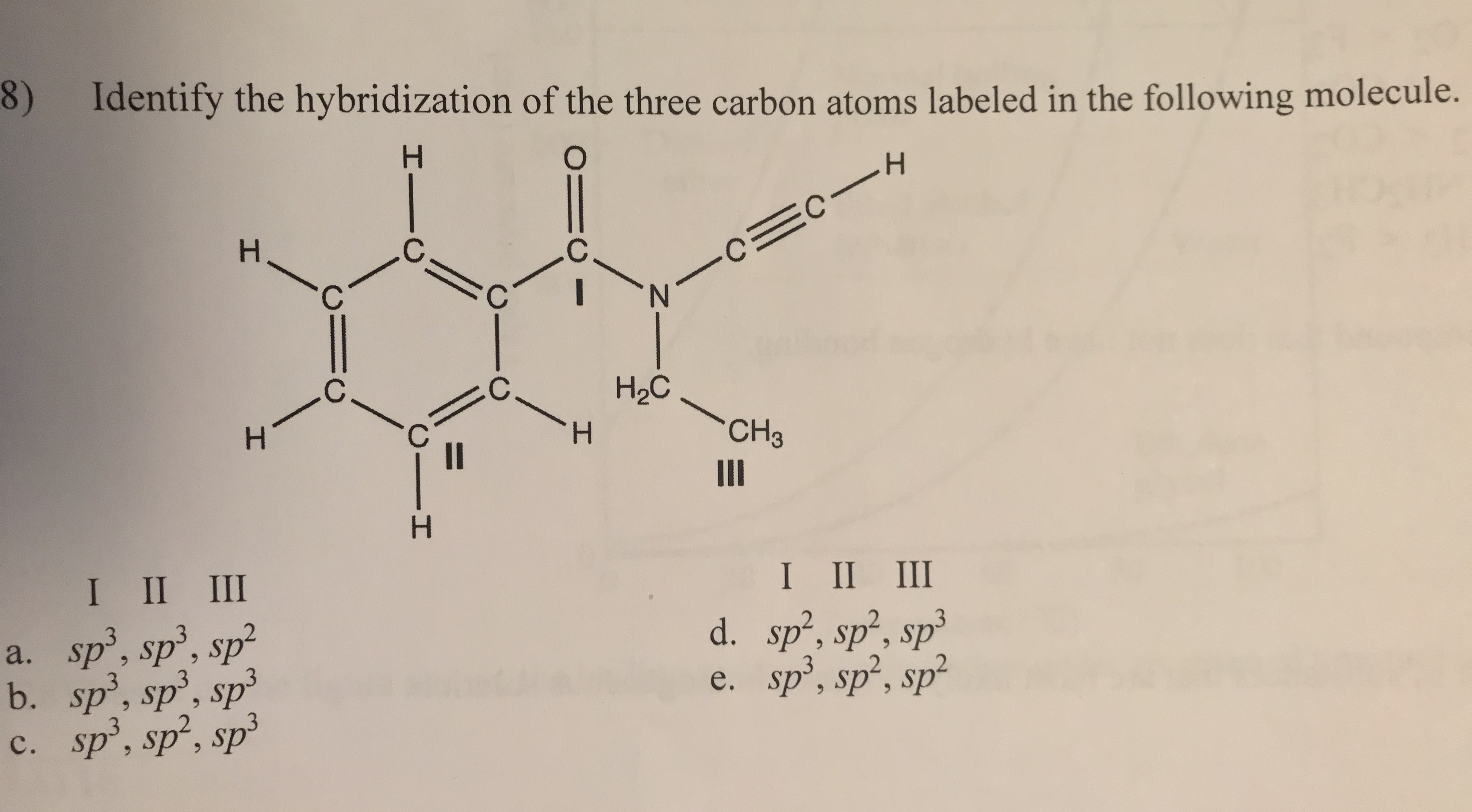
Identify the hybridization of the three carbon atoms located in the following molecule. PS - I get the first two carbons, but I am having a hard time understanding the third. (The correct answer is D) PLEASE HELP?!?
1 Answer
Jul 26, 2018
Explanation down below!!
Explanation:
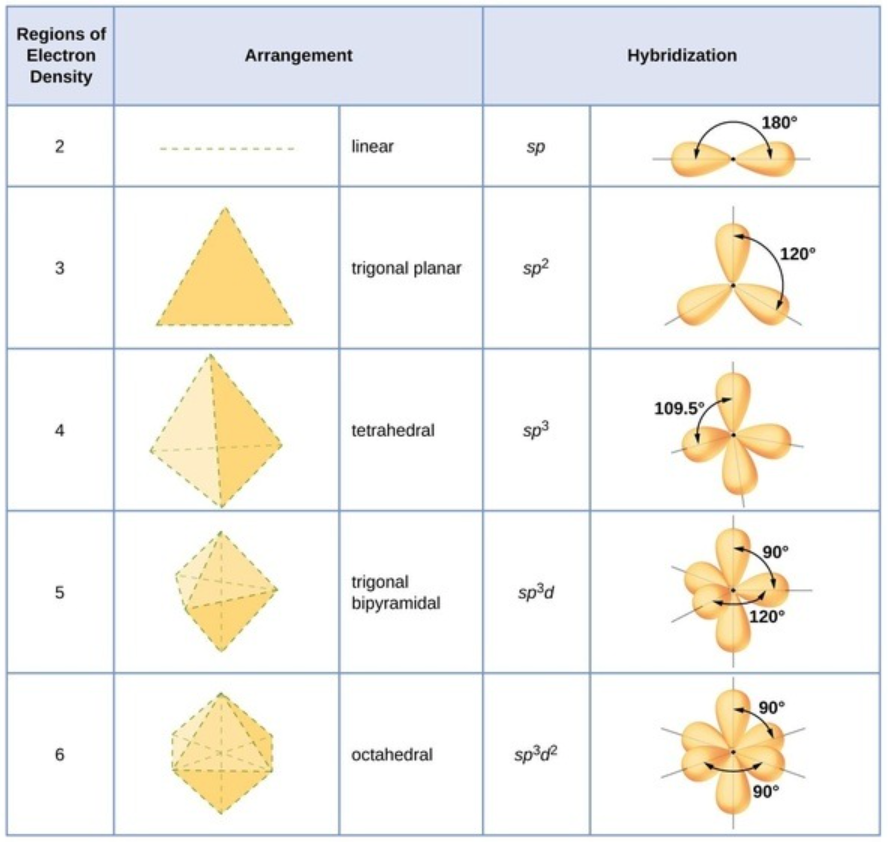 https://www.quora.com/How-does-hybridization-explain-the-shape-of-molecules
https://www.quora.com/How-does-hybridization-explain-the-shape-of-molecules
The first two carbons form covalent bonds with 3 other atoms each. Looking at the diagram above, we can see that this would form a trigonal planar arrangement seeing that each of the carbons will be surrounded by 3 other atoms and therefore 3 regions of electron density.
If we look at the third carbon, it forms 4 bonds. One of the bonds is with carbon, and the other with the 3 hydrogens. Given that is forms four separate bods, the region of electron density will be tetrahedral and the hybridization will be
Hope this helped! :D

