What happens to the configuration of the alkile halides in the SN2 mechanism?what does it mean "configuration"?
1 Answer
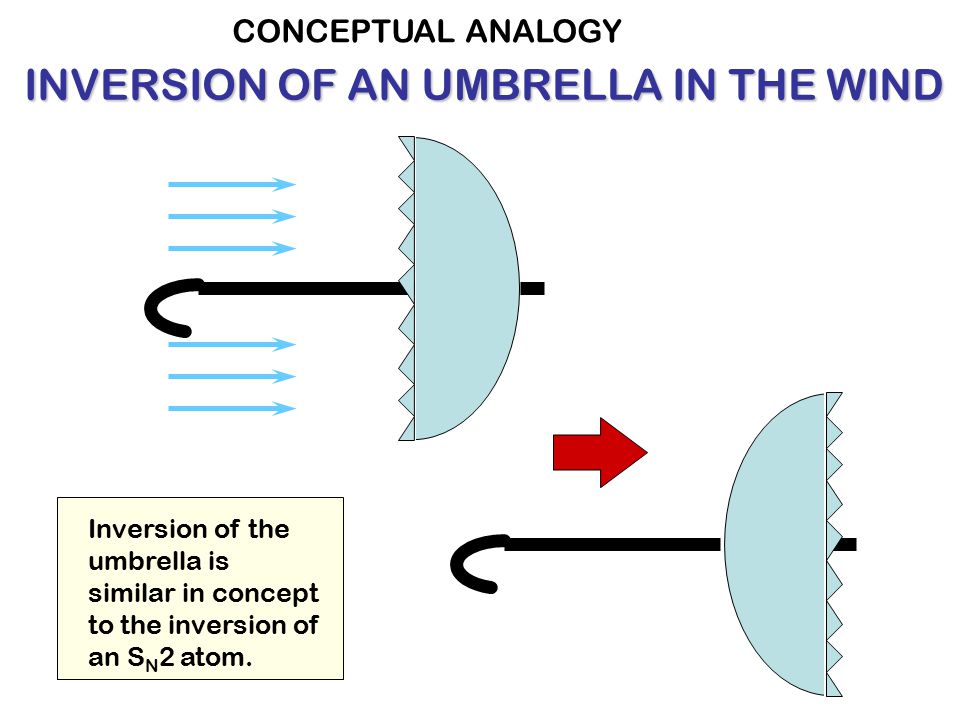
The stereometric configuration of the carbon atom under attack inverses in an
Explanation:
The halide atom (e.g., iodine) being substituted has high electron density and repel the three alkyl groups bonded to the very carbon atom away from itself. The incoming hydroxyl group attacking the same carbon atom attaches itself to the molecule on the opposite side of the leaving halide atom. The hydroxyl group is more electronegative than the halide atom and repels the carboxyl groups away from itself towards the halide atom.
 https://slideplayer.com/slide/4817246/
https://slideplayer.com/slide/4817246/
In the umbrella analogy, the umbrella fabric resembling carboxyl groups inverses during the attack. The halide atom is located at the tip of the umbrella. The incoming hydroxyl group attaches itself to the opposite side of the halide atom on the handle of the umbrella. The wind resembles electrostatic repulsion the hydroxide group exerts on the carboxyl groups and is sufficiently strong to lead to their inversion.
The stereometric configuration of the carboxyl groups in the alcohol molecule with reference to the position of the hydroxyl group is the mirror image of that of the alkali halide molecule. Thus the stereometric configuration of the carbon atom is inflected.
Reference:
"The SN2 Reaction", http://iverson.cm.utexas.edu/courses/310N/ReactMoviesFl05%20/SN2text.html

