Can someone solve these questions?( Melting and boiling chapter)
The questions are related to the diagram, no need to answer question d, Thank you in advance![![enter image source here]]() (
(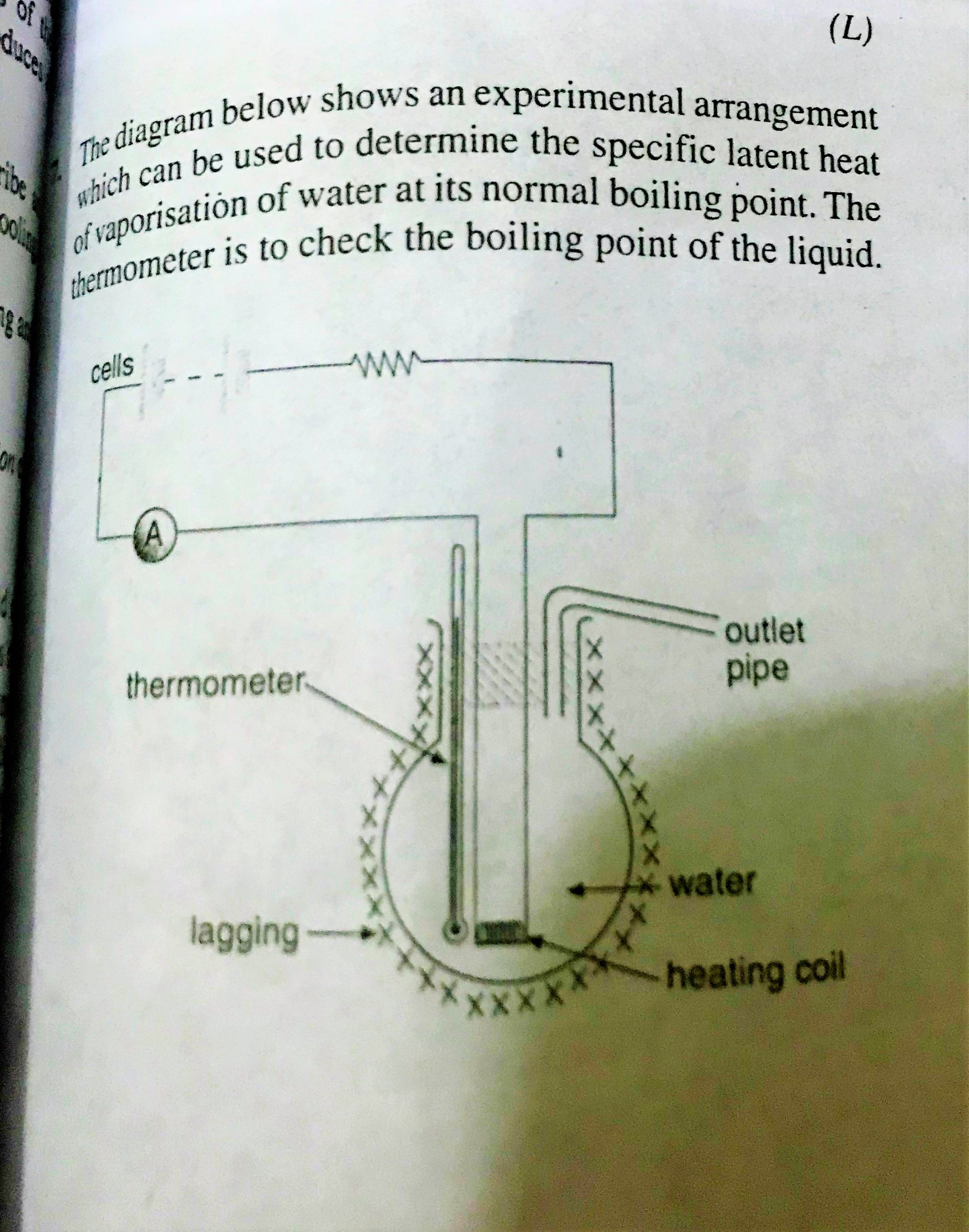 )
)
The questions are related to the diagram, no need to answer question d, Thank you in advance (
)
1 Answer
(a) Condense the water vapor coming out of the outlet pipe.
(b) Temperature will rise because the boiling point will be higher.
(c) Salt water boils at a higher temperature than pure water.
Explanation:
(a) Adding a condenser to the outlet will allow you to collect the evaporated water.
If the now condensed water drips into a beaker on a scale then the mass of the water collected in a certain time can be measured.
(b) Liquids boil at higher temperature when they are at higher pressure so the thermometer will read higher. The pressurized gas (steam) above the liquid pushes down on the surface with more force so the molecules in the liquid need more energy to escape into the gaseous state.
(c) Adding salt increases the boiling point of water (and decreases its freezing point) so the thermometer will read higher if the water is boiling.