How do we decide if a substance is paramagnetic or diamagnetic?
1 Answer
Electron spin generates a spin magnetic moment that determines whether a substance is diamagnetic or paramagnetic.
Explanation:
An electron behaves as if it were spinning on its axis. The electron bears a charge, and a charge in motion is an electric current.
We learn in physics that an electric current generates a magnetic field. So an electron behaves like a tiny magnet.

An electron has a spin magnetic moment that can line up with or against an applied magnetic field. Lining up with the field is the lower energy state. It corresponds to
If all electrons in an atom are paired, then the number of electrons with

Thus, NaCl is diamagnetic, because all spins are paired in Na⁺ and in Cl⁻.
If an atom has one or more unpaired electrons, the magnetic dipoles of the unpaired electrons will line up with an applied magnetic field. The substance will be paramagnetic.
For example, the electron configuration of nickel is
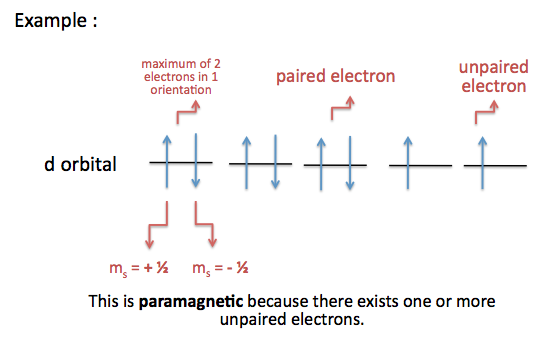
Note: an atom has other magnetic moments besides those caused by electron spin.

