Question #1e14c
1 Answer
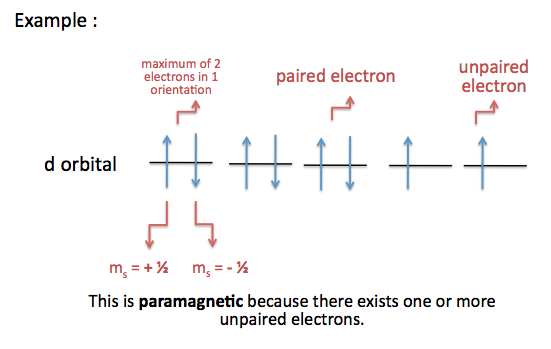
An isolated
However, an element's magnetic character does not depend solely on electron configuration, it depends on the empty orbitals that are next to the valence orbitals as well.
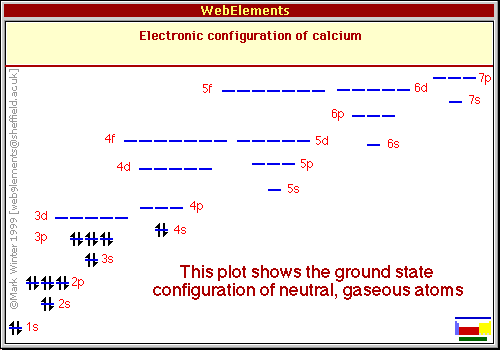
Notice that you have a vacant 3d-orbital very close in energy to the 4s-orbital. This sometimes can cause the transition of an electron from the 4s to the 3d-orbital, which causes two unpaired electrons to occupy the aforementioned orbitals and induce a paramagnetic character.
Another important aspect to take into consideration when discussing
Here's an answer detailing
http://socratic.org/questions/why-calcium-is-paramagnetic-instead-of-fully-filled-orbitals