Question #22b26
1 Answer
It depends on your definition of "rigid".
The structure of acetylsalicylic acid is
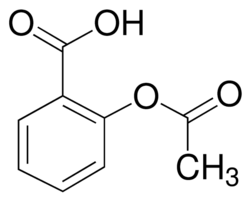
Your instructor probably expects you to say that the benzene ring and the two C=O groups with the atoms directly attached to them are rigid structures.
All double bonded atoms are unable to rotate because the π bonds prevent them from doing so. In this sense, they are "rigid".
So the 6-membered ring with alternating C-C and C=C bonds is "rigid".
The C=O carbon atoms and the C and O atoms directly attached to them form two more "rigid" groups.
But, technically, there are no rigid parts.
Every bond is continually stretching, bending, and twisting about an equilibrium position.
These motions account for the infrared spectra of molecules.

Each peak in the spectrum corresponds to a different type of motion. So the acetylsalicylic acid molecule is certainly not rigid.

