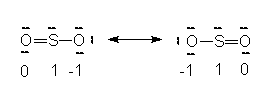The lone pair on the #S# atom, I suppose that is what you are referring to...
Taking into consideration #SO_2#'s Lewis structure - more on that here: http://socratic.org/questions/what-is-the-lewis-structure-for-so2 - the lone pair of electrons on the #S# atom plays a role in the #SO_2# molecule's its electron geomerty.
According to VSEPR theory, the shape of the #SO_2# - its electron and molecular geometries - molecule can be determined by calculating #S#'s steric and coordination numbers.
The steric number (SN) represents the number of bonds that respective atom forms with other atoms (single, double and triple bonds all count as 1) added to the number of lone electron pairs it has.
The coordination number (CN) represents the number of other atoms to which the analyzed atom is connected.
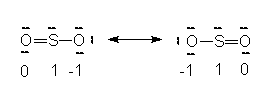
So, looking at #SO_2#'s Lewis structure (any of the three resonance structures will do), we can see that the #S# atom's #SN = 3# (2 bonds with oxygen + 1 lone pair) and its #CN = 2# (2 bonds with 2 oxugen atoms).
This means that the #SO_2# molecule's electron geometry (taking into consideration lone pairs) is trigonal planar, while its molecular geometry (without taking into consideration lone
pairs) is bent.
This is where the lone pair of electrons on the #S# atom makes a difference. SInce electrons are repelled by eachother, the electron pair will determine a bond angle of less than #120^@#, what we would see in the case of linear molecular and electron geometries.
So, #S#'s lone pair of electrons influences #SO_2#'s molecular geometry.
More on electron and molecular geometries here:
http://en.wikipedia.org/wiki/VSEPR_theory