Question #fa03c
1 Answer
Consider the structure of the
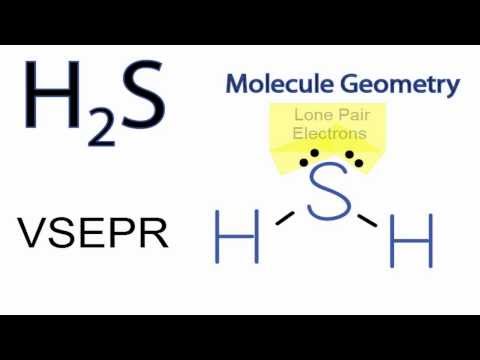
The repulsion of the two lone pairs of electrons on the sulfur atom causes the molecule to bend. The more electronegative sulfur atom attracts the bonded electrons more strongly and develops a slight negative charge, while the less electronegative hydrogen atoms develop a slight positive charge, and the molecule forms a dipole, making the molecule polar.
The molecular geometry is important in this case, as it is with water. If

