Question #e9b31
1 Answer
Mar 10, 2015
Fluorine is located in period 2, group 17 of the periodic table and has an atomic number equal to 9.
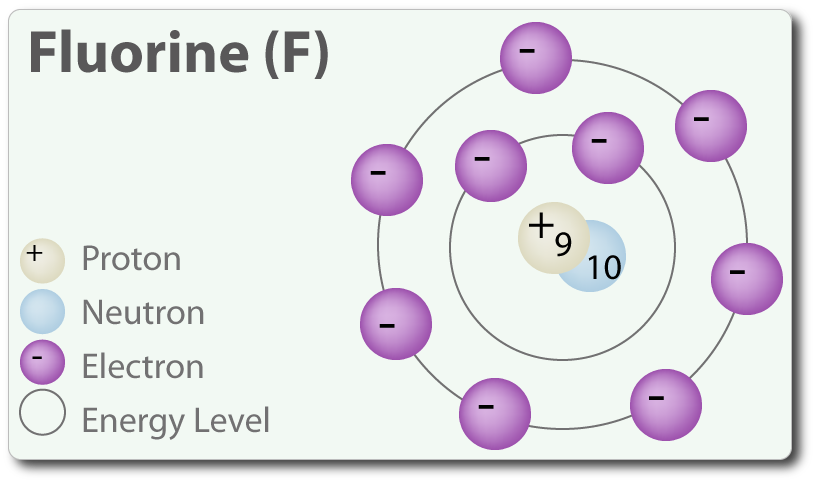
An element's atomic number represents the number of protons it has in its nucleus; as you can see in the above diagram, fluorine has 9 protons in its nucleus

In the periodic table, fluorine is located between oxygen and neon, which have atomic numbers equal to 8 and 10, respectively.

