Question #3fd4a
1 Answer
Mar 16, 2015
Phenol and resorcinol are both resistant to mild oxidation.
Hydroquinone can under go mild oxidation by ammoniacal silver nitrate or "Tollen's Reagent".
Tollen's Reagent is a solution of diammine siver(I) ions:
They can be regarded as
Phenol is :

Resorcinol is 1, 3 dihydroxybenzene:

Hydroquinone is 1, 4 dihydroxybenzene:
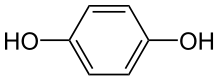
This will undergo mild oxidation to benzoquinone:

In doing so silver(I) ions are reduced to metallic silver giving the characteristic "silver mirror".
Espero que esto sea lo que pedían .

