Are there any compounds with pentavalent carbon?
1 Answer
Scientists first prepared methanium ion in 1950.
Explanation:
Methanium ion
Fluoroantimonic acid
It is a fluxional ion, in which the
In the animation above, the black ball in the middle is the carbon atom, and the red and white ones are hydrogen atoms. The blue clouds represent the electron pairs.
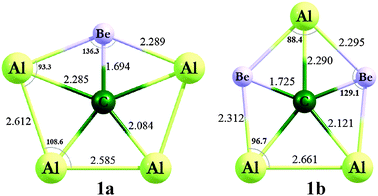
A theoretical pentacoordinate carbon
Theoretical chemists have predicted that

The bonds are not ordinary σ bonds. Instead, they involve various σ and π orbitals involving the whole molecule.
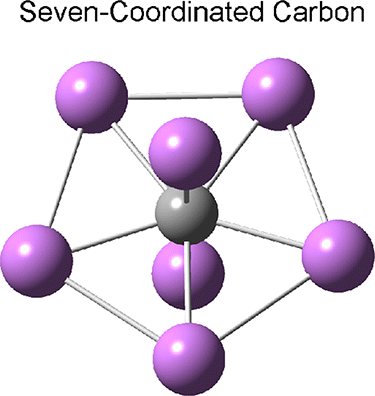
Heptacoordinate carbon?
Another theoretical possibility is