Question #a0d6e
1 Answer
Apr 24, 2015
The size of an atomic orbital is determined by the principal quantum number,
Each of these various shells describes a certain energy level and is located at a certain distance from the nucleus.
Starting from the second shell, for which n = 2, you can have various subshells located within a shell.
Three p-orbitals are located in the 2p-subshell (or in any other p-subshell, for that matter), each with a different spatial orientation.
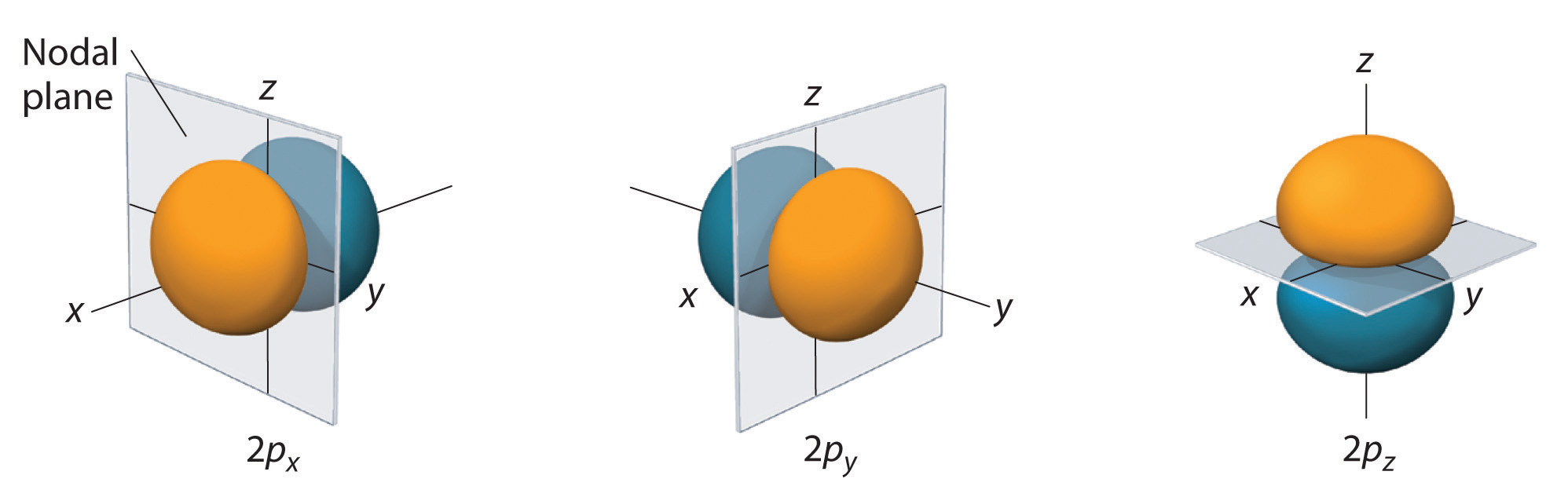
All the three p-orbitals that belong to the second shell will have the same size because they are all located at the same distance from the nucleus, i.e. the electrons that occupy them feel the same attraction from the nucleus.

