Question #d9ef1
1 Answer
London dispersion forces are attractions between temporary dipoles that form due to an unsymmetrical distribution of electrons about orbital cloud.
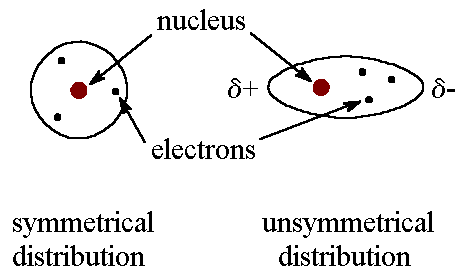
Larger molecular weight diatomic elements like iodine and bromine have higher melting/boiling points because the London dispersion forces are stronger. The explanation for this is along the lines of these atoms having more electrons and therefore form stronger temporary dipoles.
For this reason, molecular weight is often used as the rationale to explain the answer to this question. It is not quite true though. Longer carbon chains (bigger molecules) have more "atoms" of surface area and can form a number of simultaneous temporary dipoles. This results in more points of interaction and attraction between molecules.
The increase in melting/boiling point is a typical trend for any homologous series - the value increases as you add carbon atoms

