Question #141d0
2 Answers
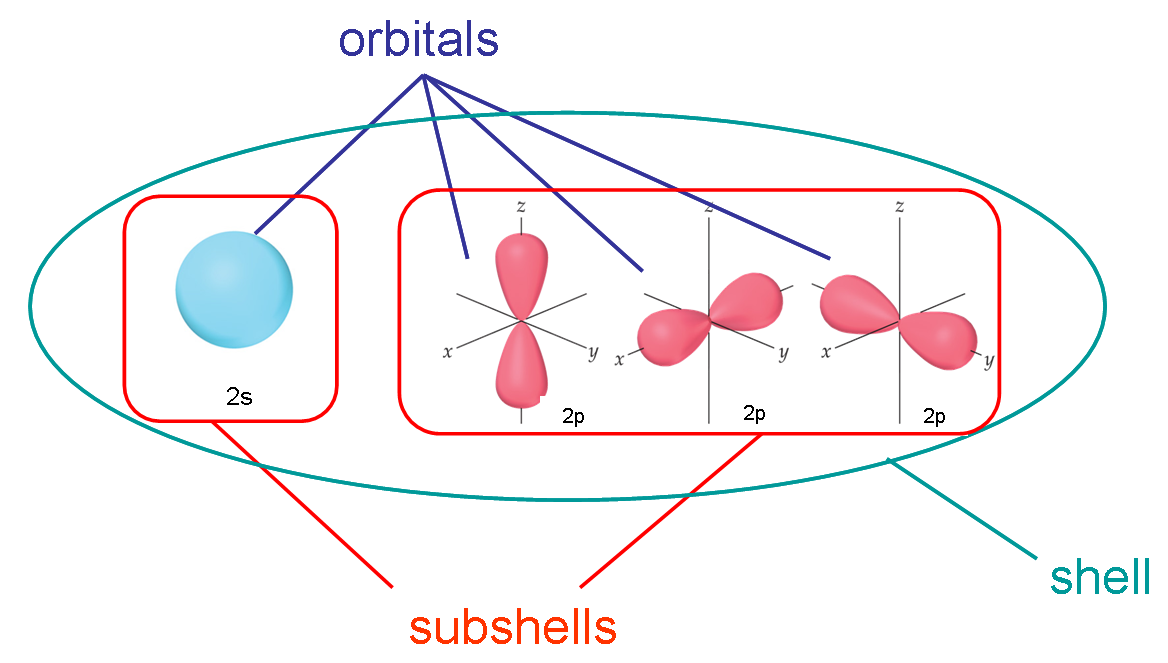
The difference between a subshell and an orbital is that, a subshell contains orbitals(at least one),
whereas, an orbital contains a maximum of two electrons.
This means that a p subshell contains three p orbitals(these are often labelled
whereas, an s subshell has only one s orbital(that is, an s subshell is equivalent to an s orbital)
So, the 2p subshell
And the 2s subshell has one 2s orbital
In general, a subshell is comprised of orbitals.
In this particular case, the 2p-subshell contains three 2p-orbitals,
If you go by number of electrons, each 2p-orbital can hold a maximum of 2 electrons. Since you have three 2p-orbitals in the 2p-subshell, the maximum number of electrons the 2p-subshell can hold is 6.
The 2s-subshell, on the other hand, only contains one orbital, the 2s-orbital, so you can say that the 2s-orbital is also the 2s-subshell, and vice versa.