Carbon uses #sp^3#, #sp^2#, and #sp# hybridization when it forms compounds.
#sp^3# Hybridization
When a carbon atom bonds to four other atoms, it mixes one #2s# and three #2p# orbitals to form four new energetically equivalent #sp^3# hybrid orbitals.
These orbitals are at angles of 109.5 ° to each other and point toward the corners of a tetrahedron.

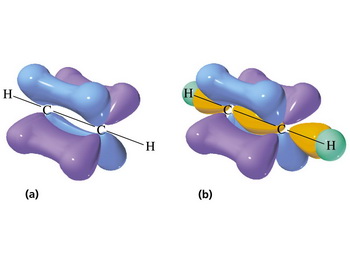
#sp^2# Hybridization
When a carbon atom bonds to three other atoms, it mixes one #2s# and two of the three #2p# orbitals to form three new energetically equivalent #sp^2# hybrid orbitals.
The #sp^2# orbitals are at angles of 120 ° to each other and point toward the corners of an equilateral triangle.
We say that they have a trigonal planar geometry.
The unhybridized #2p# orbital is perpendicular to the plane of the #sp^2# orbitals.

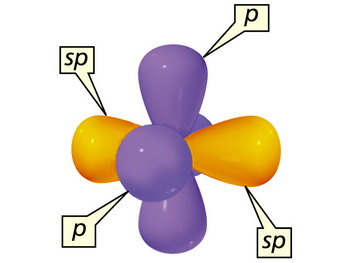
#sp# Hybridization
When a carbon atom bonds to two other atoms, it mixes one #2s# and one of the three #2p# orbitals to form two new energetically equivalent #sp# hybrid orbitals.
The #sp# orbitals are at angles of 180 ° to each other and point directly away from each other in a straight line.
We say that they have a linear geometry.
The two unhybridized #2p# orbitals are perpendicular to each other and to the #sp# orbitals.
The unhybridized p orbitals are used to make the π bonds in alkynes like ethyne.