Warning! Long answer. Here's what I get.
You have to draw the Lewis structure of each molecule, use VSEPR theory to determine its shape, and then decide whether or not the bond dipoles cancel.
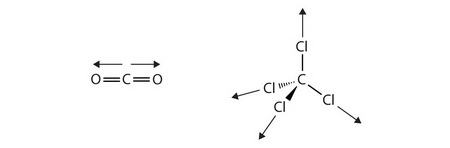
#"CO"_2# and #"CCl"_4#
(From www.peoi.org)
#"CO"_2# is a linear molecule with an #"O-C-O"# bond angle of 180°. The bond dipoles are equal and in opposite directions, so they cancel.
#"CO"_2# is a nonpolar molecule. Its strongest intermolecular forces are London dispersion forces.
#"CCl"_4# is a tetrahedral molecule with a #"Cl-C-Cl"# bond angle of 109.5°.
The two #"C-Cl"# bond dipoles in the plane of the paper have a resultant pointing to the right at an angle of 54.75° from the vertical.
The two #"C-Cl"# bond dipoles behind and in front of the paper have an equal and opposite resultant to the first.
Since the bond dipoles are equal and in opposite directions, they cancel.
#"CCl"_4# is a nonpolar molecule. Its strongest intermolecular forces are London dispersion forces.
#"CH"_2"Cl"_2#
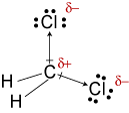
#"CH"_2"Cl"_2# has a tetrahedral shape. The two #"C-Cl"# bond dipoles have a resultant that bisects the #"Cl-C-Cl"# bond angle.
#"CH"_2"Cl"_2# is therefore a polar molecule, and its strongest intermolecular forces are dipole-dipole forces.
#"CH"_3"OH"#
#"CH"_3"OH"# has a highly polar #"O-H"# bond. The #"O"# atom has a high #δ^"-"# charge, and the #"H"# of the #"OH"# has a high #δ^+# charge.
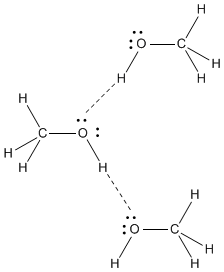
The #"O"# in one molecule is strongly attracted to the #"H"# in another molecule, and the #"H"# in one molecule is strongly attracted to the #"O"# in another molecule.
The strongest intermolecular force in #"CH"_3"OH"# is hydrogen bonding.
#"SCl"_4#
(From en.wikipedia.org)
#"SCl"_4# has a see-saw shape.
The two horizontal #"S-Cl"# bond dipoles cancel, but the downward-pointing dipoles reinforce each other.
#"SCl"_4# is a polar molecule, and its strongest intermolecular forces are dipole-dipole forces.
#"SCl"_6#
#"SCl"_6#is an octahedral molecule.
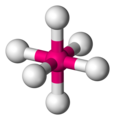
Every #"S-Cl"# bond dipole has a partner pointing in exactly the opposite direction, so all bond dipoles cancel.
#"SCl"_6# is a nonpolar molecule, so its strongest intermolecular forces are London dispersion forces.






