Question #0e7fb
1 Answer
The new volume will be equal to 117 mL.
You know that, because temperature and number of moles of gas are kept constant, you can apply Boyle's Law to determine the new volume of the gas.
According to this law, volume and pressure have an inverse relationship when temperature and number of moles are kept constant.
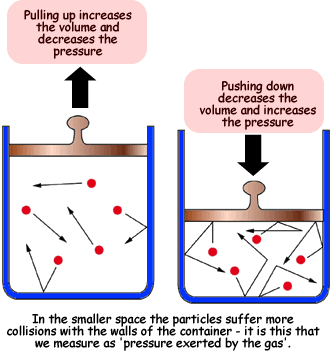
In other words, if you increase pressure, volume is going to decrease, and if you decrease pressure, volume is going to increase.
Mathematically, this is written as
You know that your pressure is increasing by 25%, so you can expect the final volume to be smaller than the initial one. The new pressure will be equal to 125% of the initial pressure
Plug your values into the equation and solve for
Rounded to three sig figs, the answer will be

