Question #8af51
1 Answer
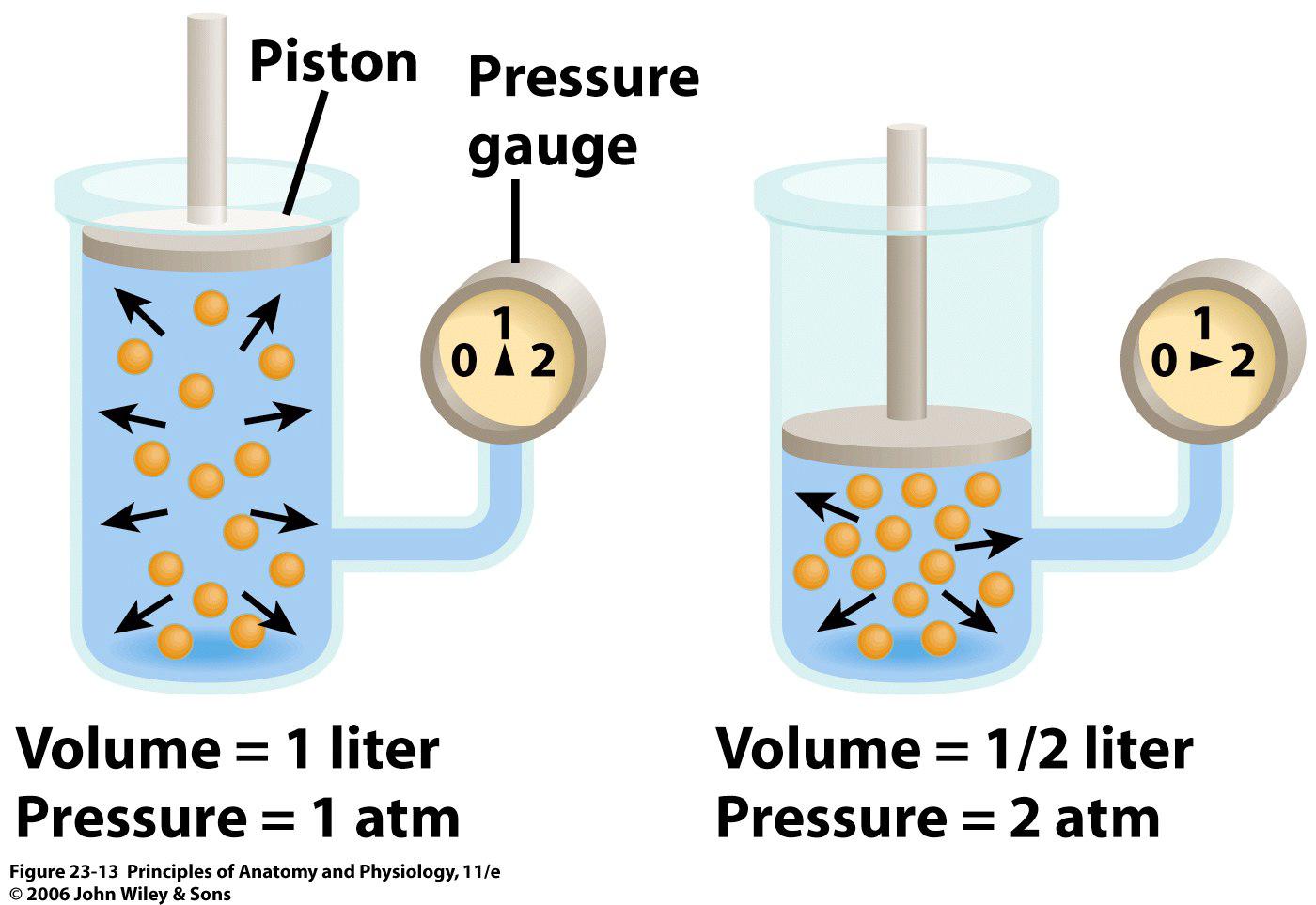
The pressure and volume of a gas have an inverse relationship when temperature is kept constant.
Assuming the the number of moles of gas is kept constant as well, i.e. you neither add, nor remove gas molecules from a container, a constant temperature implies that the pressure and volume have an inverse relationship.
In other words, if pressue increases, volume decreases. If volume increases, pressure decreases - this is known as Boyle's Law.
Mathematically, this relationship is written like this
So, if you have a gas that occupies a volume of 1 L at a pressure of 1 atm, and want to know what would its pressure be if you halved its volume, you would have
Volume got halved
This is what an inverse relationship means - when one of the parameters increases, the other parameter does exactly the opposite, i.e. it decreases.
If volume were to become 3 times bigger, then pressure would be 3 times lower.
If pressure and volume were to have a direct relationship, then that would mean that any increase or decrease in one of these parameters would also take place in the other.
As you can see, this is not the case with pressure and volume when temperature and number of moles are kept constant.

