Question #4fd9e
1 Answer
Jun 9, 2015
Because their atoms have very, very stable electron configurations.
Explanation:
Noble gases are located in group 18 of the periodic table and have extremely stable electron configurations.
With the exception of helium,
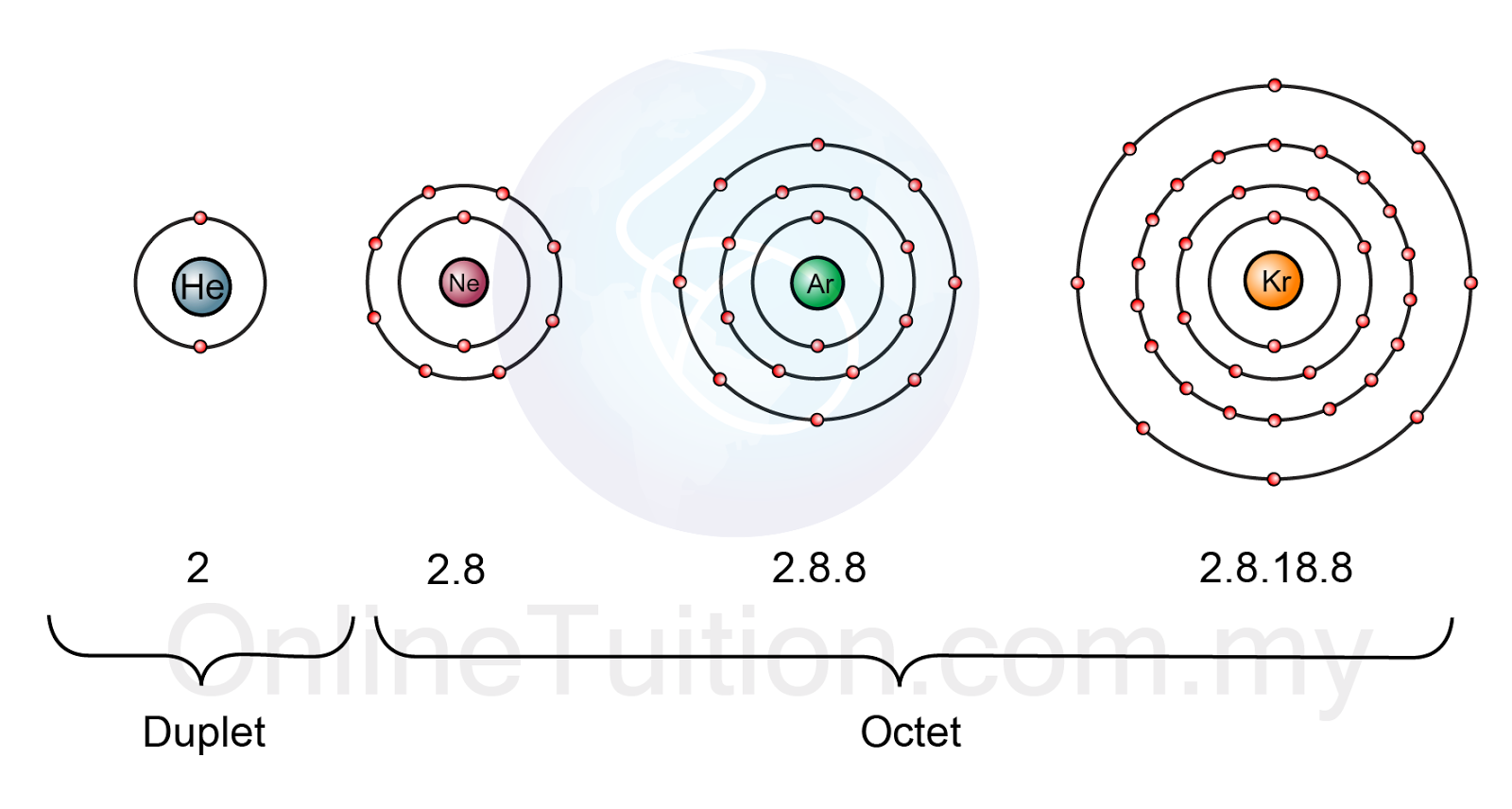 http://spmchemistry.onlinetuition.com.my/2013/10/stability-of-noble-gases.html
http://spmchemistry.onlinetuition.com.my/2013/10/stability-of-noble-gases.html
If you judge chemical reactivity by an atom's "desire" to obtain a complete octet, then noble gases would justifiably be very, very unreactive. Their atoms have no reason to react with each other in order to form, for example, diatomic molecules.
Their atoms are as stable as they can possibly be on their own, that's why noble gases are monoatomic.

