Why does B(CH3)3 violate the octet rule?
1 Answer
May 14, 2015
The octet rule is a chemical rule of thumb that states that atoms of main-group elements combine in such a way that each atom will have eight electrons in its valence shell.
The Lewis structure of
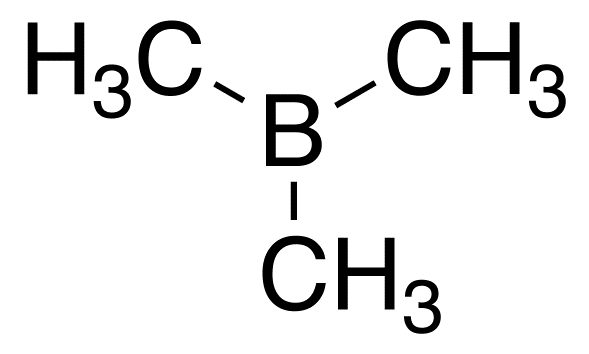
We see that the molecule has only six valence electrons around the
This is a violation of the octet rule.

