Question #a5da3
1 Answer
The energy of the reactant(s) is
Explanation:
The information given to you describes an exothermic reaction because the activation energy of the reverse reaction is smaller than the activation energy of the forward reaction.
This implies that the products are lower in energy than the reactants. As a result, you should expect the energy of the reactants to exceed the energy of the products.
A generic potential energy diagram for an exothermic reaction looks like this
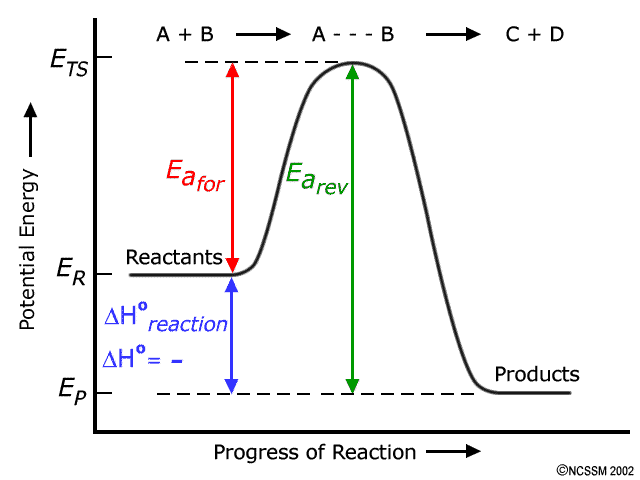
I won't go into the details about what activation energy and threshold energy are, but you can read more on that here:
So, in your case, the forward reaction has an activation energy equal to
The reverse reaction has an activation energy equal to
Moreover, you know that the nergy level of the products is equal to
This means that the threshold energy of the reaction, i.e. the average kinetic energy that molecules need to react, can be determined by adding the activation energy of the reverse reaction to the energy of the products.
So, in order to get a reaction, your molecules need to have an average kinetic energy of +329 kJ. Since you know what the activation energy of the forward reaction is, you can determine the energy of the reactants by
The enthalpy change of reaction will be negative, since the energy of the products is lower than the energy of the reactants.
The minus sign symbolizes the fact that heat is released by the reaction.

