You should see the following peaks.
#"3100-3000 cm"^(-1) "(w)"color(white)(XXXXXXXXXXXXXl) "Aromatic C-H stretch"#
#"2960 and 2870 cm"^(-1) "(m)" color(white)(XXXXXXXXXXXl)"Methyl C-H stretch"#
#"1730-1715 cm"^(-1) "(s)" color(white)(XXXXXXXXXXXXXX)"Conjugated ester C=O stretch"#
#"1600-1585 cm"^(-1) "(m) and 1500-1400 cm"^-1 "(m)"color(white)(X)"Aromatic C-C stretch"#
#"1380 cm"^(-1) "(w) and 1260 cm"^(-1) "(m)" color(white)(XXXXXXX)"Methyl C-H bend"#
#"1300-1000 cm"^(-1)"(s) (three bands)"color(white)(XXXXXXX) "C-O stretch"#
#"750-700 cm"^(-1) "(s) and 710-690 cm"^(-1) "(m)" color(white)(XXXX)"Aromatic C-H 'out-of-plane' bend"#
The structure of methyl benzoate is
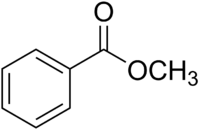
It has a monosubstituted benzene ring, an ester group, and a methyl group.
1. #" C-O"# stretching
The strongest peak should be the ester #"C=O"# stretch.
This normally appears at #"1750-1735 cm"^-1#, but conjugation with the ring shifts the peak to #"1730-1715 cm"^-1#.
There should also be one medium and two strong bands in the region from #"1300-1000 cm"^-1#.
The two strong bands arise from the symmetric and antisymmetric #"C-O"# stretches of the ester #"COO"# group, and the weaker band is the ether #"CO"# stretch of the #"OCH"_3# group.
2. #"C-C"# Stretching
The benzene ring has characteristic medium-strength #"C-C"# stretching bands in the #"1600-1585 cm"^(-1)# and #"1500-1400 cm"^(-1)# regions.
3. #"C-H"# bending
The monosubstituted phenyl group has characteristic strong bending vibrations at #"750-700 cm"^(-1)# and #"710-690 cm"^(-1)#.
The methyl group usually shows a weak band at #"1380 cm"^(-1)# and a medium band at #"1260 cm"^(-1)#.
4. #"C-H"# Stretching
The aromatic #"C-H"# stretch is usually weak and occurs at #"3100-3000 cm"^-1#.
The aliphatic #"CH"_3# group shows stronger symmetric and antisymmetric stretches at #"2960 cm"^(-1)# and #"2870 cm"^(-1)#.
Here's the actual IR spectrum of methyl benzoate.
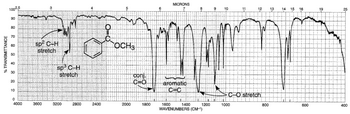
(from chemistrytextbookcrawl.blogspot.com)




