How is infrared spectroscopy used to identify the presence of organic compounds in water samples?
1 Answer
Sep 12, 2015
It's not.
Picking the appropriate solvent is important so that we know that the peaks we see are from the compound we're analyzing. Water has an IR spectrum with strong peaks in areas where we don't want to see it.
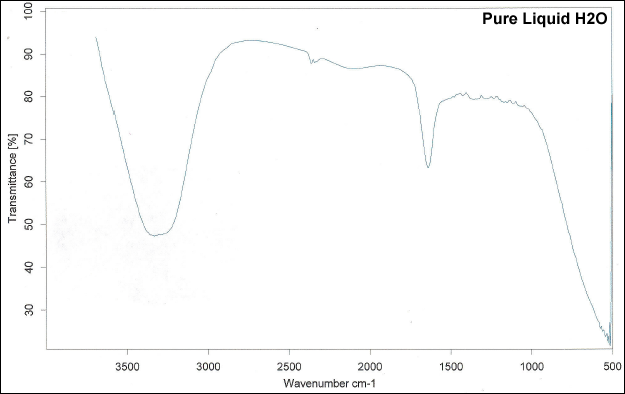
All alcohols, amines, and carboxylic acids, among other similar functional groups, have peaks in the
A much better solvent is carbon tetrachloride
Some other good solvents are acetone, diethyl ether, etc.

