Question #6902a
1 Answer
A and C are not tautomeric pairs.
Explanation:
Keto–enol tautomerism is a chemical equilibrium between a the carbonyl group of an aldehyde or ketone and its enol (an alcohol on a double bonded carbon).
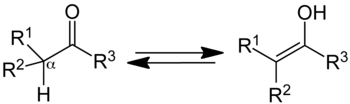
The equilibrium involves the movement of an alpha hydrogen to the carbonyl oxygen and the movement of electrons to form the
(A) 3-Methylpentan-2- one and 3-methylpentan-3-ol
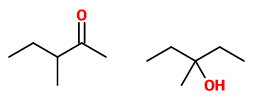
These are not tautomers. The first is a ketone (which can form an enol), but the second is an alcohol.
(B) Ethenol and ethanal
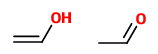
These are a tautomeric pair. The second is an aldehyde, and the first is its enol.
(C) Propan-2-ol and prop-1-en-2-ol
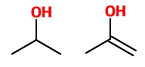
These are not tautomers. The second is an enol, but the first is an alcohol.
(D) Pentane-2,4-dione and pent-3-en-4-ol-2-one
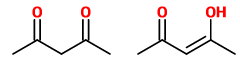
These are a tautomeric pair. The first is a ketone and the second is its enol.

