Question #df47e
1 Answer
Here's how I would approach it.
A) From the name, here is my train of thought:
- The molecule is a trans isomer (as opposed to cis)
- There are six things interacting with manganese (tetra = 4, di = 2, 4 + 2 = 6)
- Six things bound = coordination number of 6, which corresponds to an octahedral geometry for
#"Mn"^(3+)# - Since there are only two bromine ligands (dibromo) but four water ligands (tetraaqua), and since a trans structure is required, either all four equatorial ligands are the same or two of them are different.
- As a result, two equatorial ligands (of four) must be different, or the two bromines are axial.
Therefore, you should get:
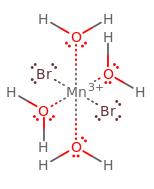
or
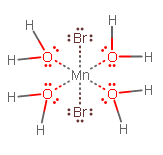
See how the bromine ligands are across from each other? trans = across (cis = same side). If you want the cis isomer, simply swap the position of one equatorial water with another equatorial bromide, or swap the position of one equatorial water with one axial bromide.
B) I would start from the parent compound.
You see that it says "pentane", which is a five-carbon alkane. Where are the methyl groups? They're on carbons 2 and 3, hence "2,3-dimethyl". (It's not that there are two methyl groups on each of those two carbons; there are two total, and one each is on those two carbons. )
So, it is just this:
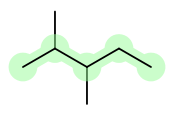
I've highlighted the pentane backbone.

