What is the difference between nodal surfaces and nodal planes?
1 Answer
A nodal surface is also called a radial node, which is a hollow spherical region in which electrons cannot be. A nodal plane is also called an angular node, which is either a plane where electrons cannot be, or a conic surface (
Radial nodes (or nodal surfaces) can be found using the principal quantum number
-
#n# goes as#1, 2, 3, . . . , N# where#N# is an integer, and#n# tells you the energy level. -
#l# goes as#0, 1, 2, . . . , n-1# , and#l# tells you the shape of the orbital.#l# is#0# for an#s# orbital,#1# for a#p# orbital,#2# for a#d# orbital, etc.
For instance, for a
#l = 0# #n - l - 1 = 1 - 0 - 1 = \mathbf(0)# radial nodes.
For a
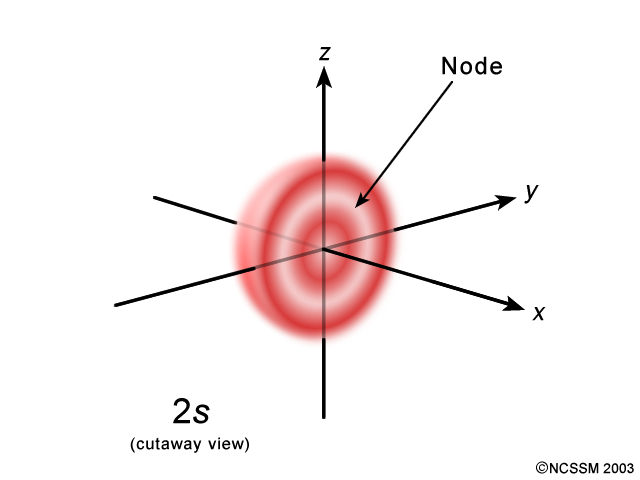
#l = 0# again#n - l - 1 = 2 - 0 - 1 = \mathbf(1)# radial node.
We can see the single radial node here as a white hollow sphere's cross-section:
Angular nodes (or nodal planes) can be found simply by determining
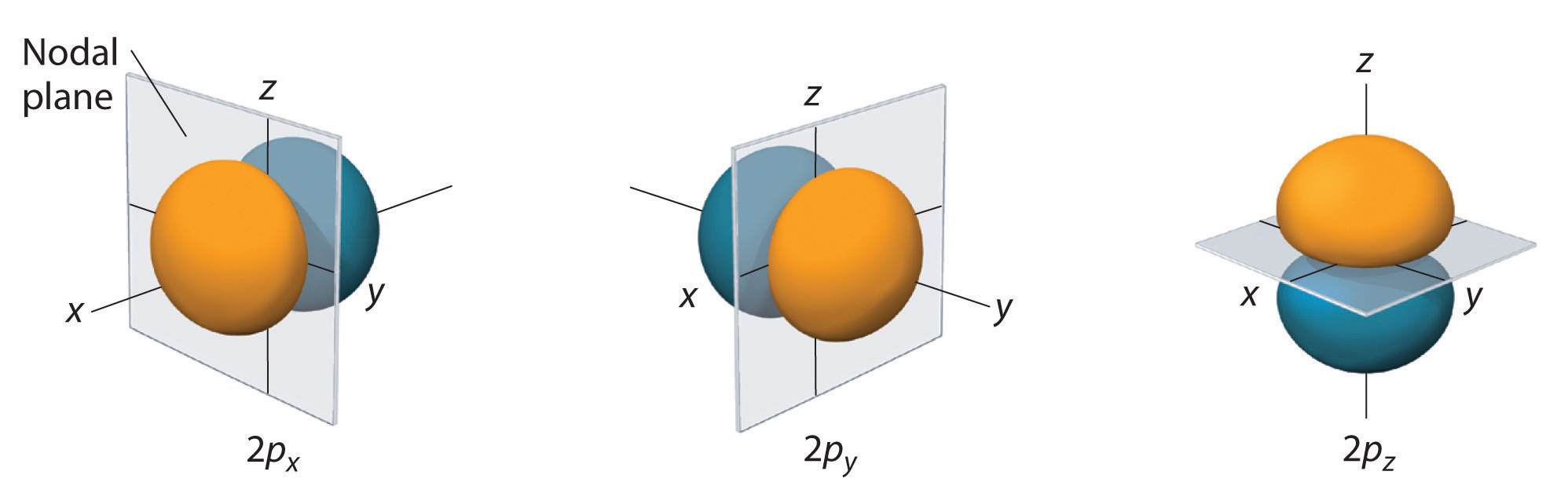
On the other hand, a


