Question #ccd80
1 Answer
Nitric acid,
Explanation:
The idea here is that you need to be familiar with the definition of an oxoacid, sometimes called oxyacid.
An oxoacid is simply an acid that contains oxygen,
In your case, the oxoacid is said to contain one atom of nitrogen,
Right from the start, you know that the hydrogen atom must be bonded to one of the three oxygen atom, since this is needed in order to make it acidic, i.e. ready to jump off in aqueous solution.
All three oxygen atoms are bonded to the nitrogen atom.
At this point, you should be able to recognize the fact that you're dealing with nitric acid,
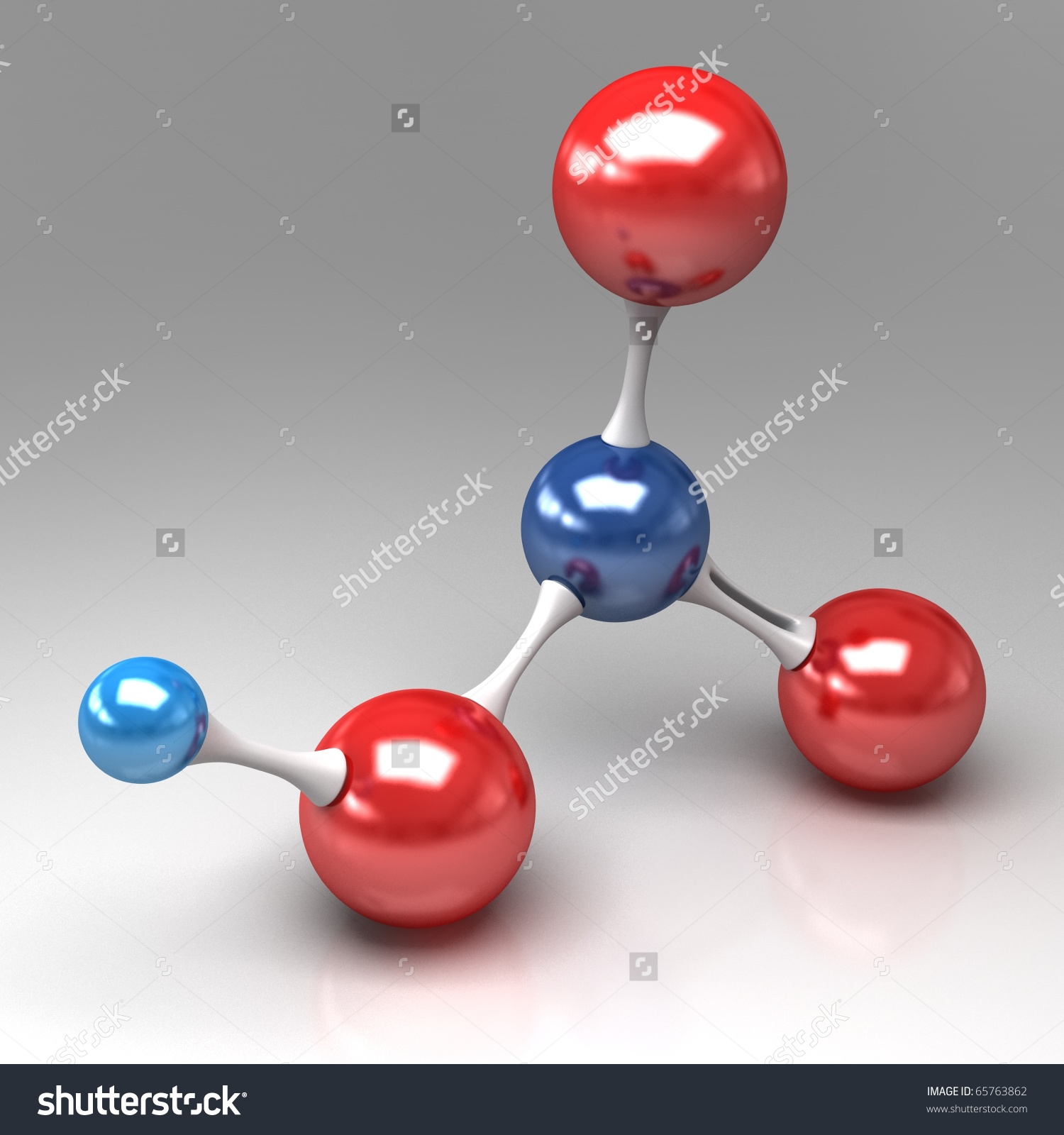
The nitrogen atom is shown here in dark blue, the oxygen atoms in red, and the hydrogen atom in light blue.
Nitric acid is a strong acid that dissociates completely in aqueous solution to form hydronium cations and nitrate anions
#color(red)("H")"NO"_ (3(aq)) + "H"_ 2"O"_ ((l)) -> "H"_ 3"O"_ ((aq))^(color(red)(+)) + "NO"_(3(aq))^(-)#

