Question #93980
1 Answer
You have to find the rate at which the enzyme by itself catalyzes the reaction without the inhibitor. Once you find the final reaction rate along with the inhibitor, calculate the difference to discover the inhibitor's effect on the reaction.
Explanation:
An enzyme by itself tends to speed up reactions, and it is always better than with inhibitors. You find the reaction rate with the enzyme by itself to serve as a comparison to the reaction rate with the inhibited enzyme.
With just the reaction rate of the inhibited enzyme, how can you conclude whether it has inhibited a lot of the reaction or only a little?
If the decrease in rate of action is significant, you can be assured that the inhibitor prevents the catalyzation of the reaction. If the decrease is small, the inhibitor may not be enough to prevent catalyzation of the reaction.
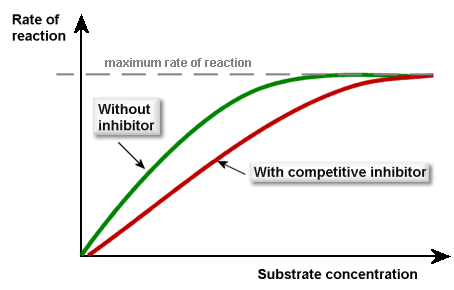
You need the initial rate of reaction to determine this difference in reaction rate, though please remember maximum rate of reaction remains same, even in presence of copetitive inhibitor, when substrate concentration increases.

( )
)

