Question #f473f
1 Answer
Here's what I got.
Explanation:
As you know, four quantum numbers are used to describe the position and spin of an electron in an atom.
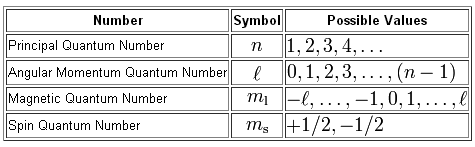
A good starting point here will be chlorine's electron configuration, which looks like this - I'll use the noble gas shorthand notation
#"Cl: " ["Ne"] 3s^2 color(red)(3)p^5#
As you can see, chlorine's outermost electrons are located in its 3p-subshell. Four of these five electrons are located in the
Let's try to write a quantum number set for the electron located in the
The principal quantum number,
The angular momentum quantum number, which gives you the subshell in which the electron is located, takes values that range from
#l=0 -># the s-subshell#l=1 -># the p-subshell#l=2 -># the d-subshell
#vdots#
In your case, the electron is located in the p-subshell, you know that you have
The magnetic quantum number,
#m_l = -1 -># the#p_x# orbital#m_l = color(white)(-)0 -># the#p_y# orbital#m_l = color(white)(-)1 -># the#p_z# orbital
Since we';re looking for the magnetic quantum number of an electron located in the
Finally, the spin quantum number, which gives you the spin of the electron, can only have two possible values
#m_s = =1/2" "# or#" "m_s = -1/2#
Since the electron occupies the orbital by itself, you can attribute to it a spin magnetic number of
This means that the quantum number set that describes the position and spin of the electron located in chlorine's
#color(green)(|bar(ul(color(white)(a/a)color(black)(n = 3, l = 1, m_l = 1, m_s = +1/2)color(white)(a/a)|)))#

