Question #51305
1 Answer
Sep 29, 2016
Here's how I would do it.
Explanation:
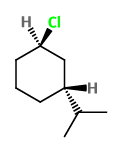
The correct name for the compound is trans-1-chloro-3-isopropylcyclohexane.
Draw a structure for cyclohexane.
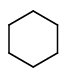 1
1
Put a wedge and a dash on each of
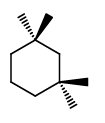 2
2
The two groups are trans to each other. Put the chlorine atom on a wedge at
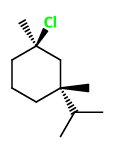 3
3
Put
 4
4
Now you have a perspective formula for trans-1-chloro-3-isopropylcyclohexane.

