How many electrons in magnesium share the quantum numbers #l=0# and #m_l = 1# ?
1 Answer
Zero.
Explanation:
As you know, four quantum numbers are used to describe the position and spin of an electron in an atom.
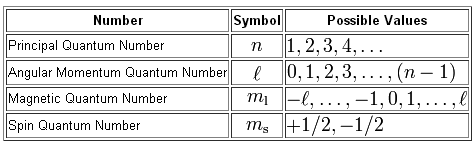
Your goal here is to identify how many electrons located in an atom of magnesium,
#l = 0" "# and#" " m_l = 1#
Start by writing the complete electron configuration for a neutral atom of magnesium. Magnesium is located in period 3, group 2 of the periodic table and has an atomic number equal to
This means that a neutral magnesium atom will have a total of
#"Mg: " 1s^2 2s^2 2p^6 3s^2#
Now, the angular momentum quantum number,
# l = 0 -># the s-subshell#l = 1 -># the p-subshell#l=2 -># the d-subshell#l =3 -># the f-subshell
#vdots#
In your case, the value
Now, the magnetic quantum number,
#m_l = -l, -(l-1), ..., -1, 0 , 1, ..., (l-1), l#
The only possible value for
#m_l = 0 -># corresponds to the s-orbital
You can thus say that no electrons share the quantum numbers
You will never find an atom in which an electron has the quantum number
ALTERNATIVE INTERPRETATION
Just in case you had to determine how many electrons share the quantum number
If you're looking for electrons that have
#1s^2 -># two electrons located in the s-subshell of the first energy level#2s^2 -># two electrons located in the s-subshell of the second energy level#3s^2 -># two electrons located in the s-subshell of the third energy level
Therefore, a total of six electrons have
Now, in a magnesium atom,
More specifically, you have
#m_l = -1 -># the#p_x# orbital#m_l = color(white)(-)0 -># the#p_y# orbital#m_l = color(white)(-)1 -># the#p_z# orbital
A magnesium atom has the p-subshell of the second energy level completely filled with electrons, which means that the
Therefore, a total of two electrons have

