Which dd orbital is specified by Y(theta,phi) = (5/(8pi))^(1//2) (3cos^2theta - 1)Y(θ,ϕ)=(58π)1/2(3cos2θ−1)?
1 Answer
Your angular wave function looks off. Every wave function
- orthogonal (
int_"allspace" psi_i^"*"psi_jd tau = 0∫allspaceψ*iψjdτ=0 wherei ne ji≠j ) - normalizable (
int_"allspace" psi_i^"*"psi_id tau = 1∫allspaceψ*iψidτ=1 )
and yours is not. The correct
color(blue)(Y_(2)^(0)(theta,phi) = (5/(16pi))^"1/2" (3cos^2theta - 1))Y02(θ,ϕ)=(516π)1/2(3cos2θ−1)
In this case, what we have here is ONLY the angular component of the wave function for the
The full wave function in spherical harmonics is written as the product of the radial and angular components:
\mathbf(psi_(nlm_l)(vecr,theta,phi) = R_(nl)(vecr)Y_(l)^(m_l)(theta,phi))
For instance, the full wave function for the
psi_(3d_(z^2)) = psi_(320)(vecr,theta,phi) = R_(32)(vecr)Y_(2)^(0)(theta,phi)
= 1/(81sqrt(6pi))(Z/(a_0))^"3/2" ((Zr)/(a_0))^2e^(-Zr"/"3a_0) (3cos^2theta - 1), where
Z is the atomic number anda_0 is the Bohr radius (5.29177xx10^(-11) "m" ).
This, you would see is quite different and much more complicated.
You did not include
Generally they look like dumbbells along the z-axis with a torus (donut) on the xy-plane. The torus has the OPPOSITE sign to the lobes, and the lobes have the SAME sign as each other.
The
- The
3d_(z^2) hasn - l - 1 = 3 - 2 - 1 = 0 radial nodes andn - 1 = 2 total nodes. Thus, it has two angular nodes.- The
4d_(z^2) hasn - l - 1 = 4 - 2 - 1 = 1 radial node andn - 1 = 3 total nodes. Thus, it has two angular nodes as before.- The
5d_(z^2) hasn - l - 1 = 5 - 2 - 1 = 2 radial nodes andn - 1 = 4 total nodes. Thus, it has two angular nodes as before.- The
6d_(z^2) hasn - l - 1 = 6 - 2 - 1 = 3 radial nodes andn - 1 = 5 total nodes. Thus, it has two angular nodes as before.
So you can see the pattern that the
For instance, here is a picture of the
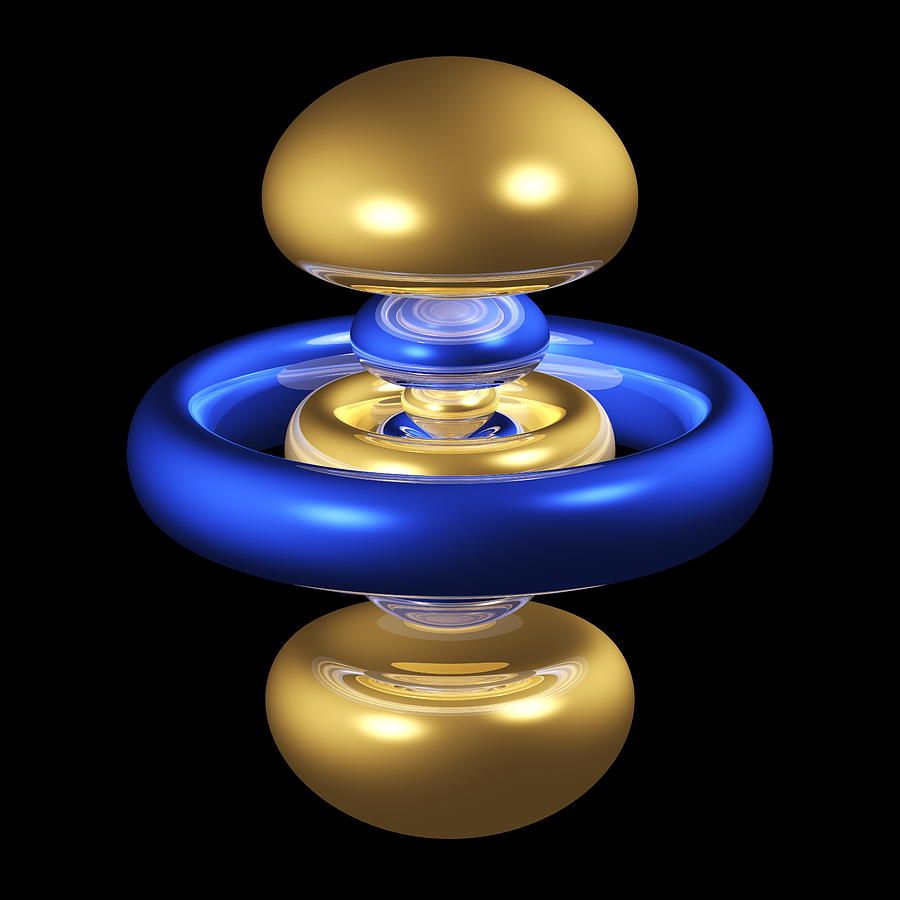
Now compare that to the
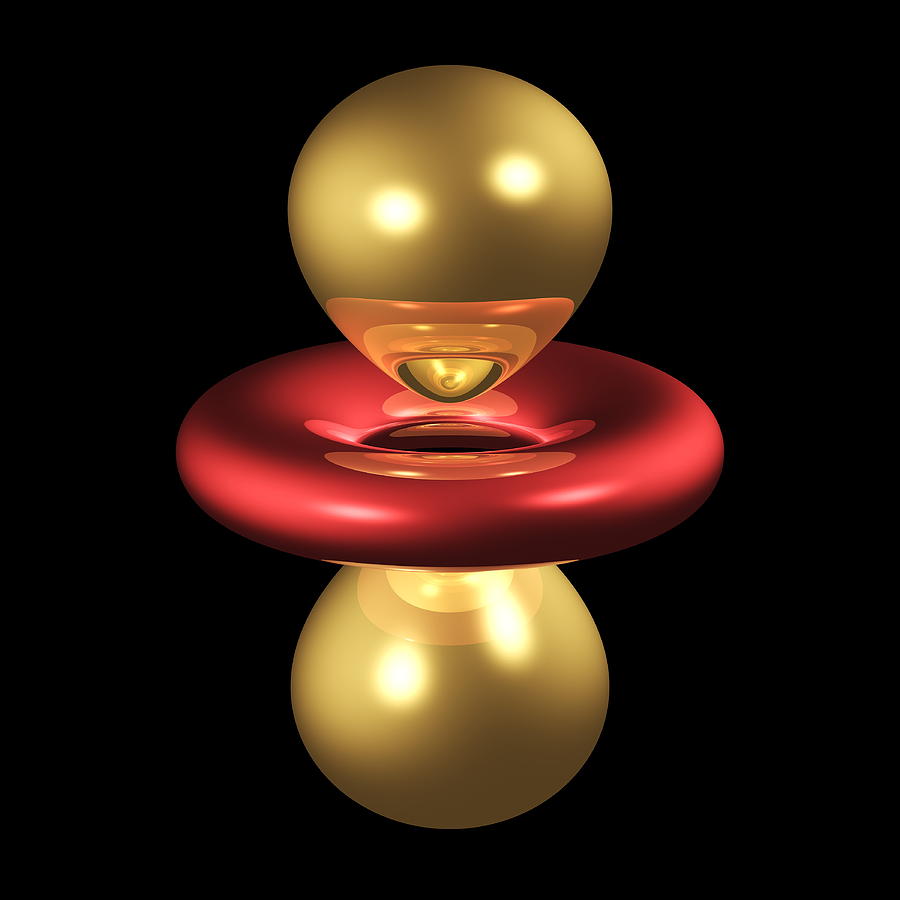
You should notice that the

