Question #4a5cf
1 Answer
Alpha radiation.
Explanation:
Polonium-212 undergoes alpha decay to produce lead-208, which means that it gives off alpha radiation, i.e. alpha particles.
An alpha particle,
- two protons
- two neutrons
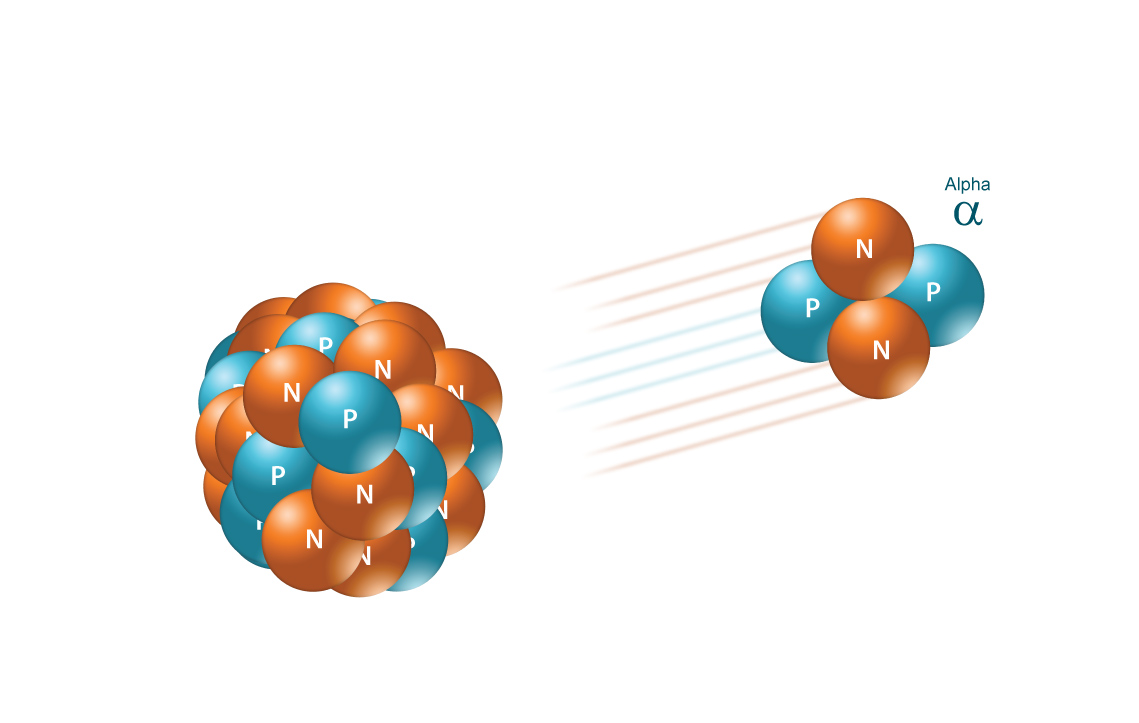
When a radioactive nuclide emits an alpha particle
- its mass number will decrease by
#4# The mass number,
#A# , is given by the number of protons and neutrons, and so
#A = "2 protons " + " 2 neutrons" = 4#
- its atomic number will decrease by
#2# The atomic number,
#Z# , is given by the number of protons, and so
#Z = "2 protons" = 2#
Now, the two elements that you're working here with have
#"Po: " {(A = 212),(Z = 84) :}" "# and#" " "Pb: " {(A = 208), (Z = 82) :}#
This confirms that you're dealing with an alpha decay because the balanced nuclear equation that describes this process looks like this
#""_ (color(white)(1)color(darkgreen)(84))^color(blue)(212)"Po" -> ""_ (color(white)(1)color(darkgreen)(82))^color(blue)(208)"Pb" + ""_ color(darkgreen)(2)^color(blue)(4)alpha#
Notice that mass and charge are conserved, since
#color(blue)(212 = 208 + 4) -># conservation of mass
#color(darkgreen)(84 = 82 + 2) -># conservation of charge

