What are the nodal planes of the #d_(x^2-y^2)# orbital?
1 Answer
Jun 29, 2016
The nodal planes of the
Explanation:
A nodal plane or nodal surface of a wave-function is an area where there is zero displacement; zero electron density.
Example:
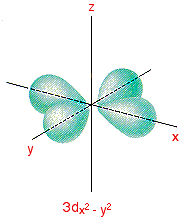
Since the


