Question #a306c
1 Answer
The product depends on which reactant is in excess.
Explanation:
The aldol condensation
An aldol condensation is usually a base-catalyzed reaction in which an aldehyde or ketone with α-hydrogens reacts with a carbonyl compound to form
a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.
For example
You can find the mechanism in this Socratic answer.
In this problem, 4-hydroxybenzaldehyde is the aldehyde and cyclopentanone is the ketone with the α-hydrogen.
If there is excess cyclopentanone
There will be only enough 4-hydroxybenzaldehyde to react at
The major product will be 2-(4-hydroxybenzylidene)cyclopentanone.
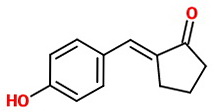
The product is probably a mixture of (
If there is excess aldehyde
Carbon 5 of the cyclopentanone still has α-hydrogens at
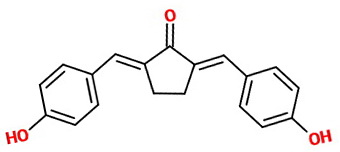
The product is probably a mixture of (

