What is the aldol condensation mechanism?
1 Answer
Aldol condensation is the reaction of a ketone with an aldehyde in strong base on high heat.
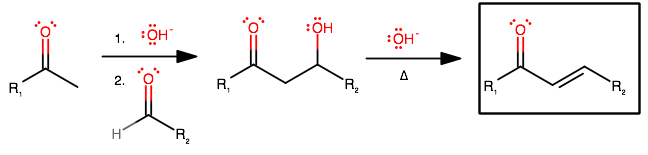
The mechanism goes like this:
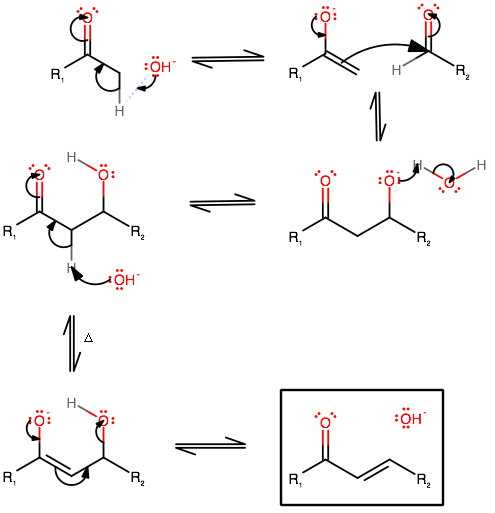
- First, the strong base (
#"OH"^(-)# ) acquires a proton from the#alpha# -carbon. This generates the enolate to some extent, and sets the substrate up for the base-catalyzed aldol reaction. - The enolate is then used as a nucleophile to attack the aldehyde in an
#"S"_N2# fashion. Remember that a new#"C"-"C"# bond is created here, so you must count your carbons and make sure of that. -
A proton transfer occurs from the
#"H"_2"O"# that formed in step 1 to protonate the oxide.This forms the
#beta# -hydroxyketone intermediate (or#beta# -hydroxyaldehyde if#R_1# is#"H"# ). -
Another
#"OH"^(-)# acquires an#\mathbf(alpha)# -proton , just as in step 1, generating an enolate. This is upon additional heating, and starts the dehydration portion of the aldol condensation, which is basically the second half of the reaction.The electrons didn't flow to the right because the
#"OH"# is electron-donating, which decreases the electropositivity of the carbon adjacent to#R_2# . Instead, some "momentum" from forming the enolate is necessary to proceed. -
The
#"OH"# is eliminated, forming the final product: an#\mathbf(alpha,beta)# -unsaturated ketone (or aldehyde, if#R_1 = "H"# ).

