Does basicity always vary inversely with ionic atomic radius???
1 Answer
Not necessarily... It's only a guideline, and one that easily gets confusing. So it's not a good guideline to try to make sense of.
We need to determine what "basicity" means. You did not specify either Lewis or Bronsted basicity, which both make a difference.
Take
BRONSTED BASICITY
Bronsted basicity generally depends on the ability to polarize a proton's bond with another atom, weakening the bond and promoting the Bronsted base's acceptance of that proton.
When you place
- It is smaller, making its electron density, well, denser, making it less polarizable by another species. Thus, it is a harder base.
- It is more electronegative, making it more polarizing towards the proton on water than
#"I"^(-)# is on water.
That allows a stronger hydrogen-bonding interaction than
(Indeed, we say that
LEWIS BASICITY
Lewis basicity may depend on the following factors:
- electronegativity
- solvent effects (hydrogen-bonding, for example)
- strength of the conjugate acid's
#"H"-"A"# bond(s), and the trade-off you get upon trying to make that bond. - and probably other stuff.
In terms of Lewis basicity, since
That increases its effectiveness as a Lewis base.
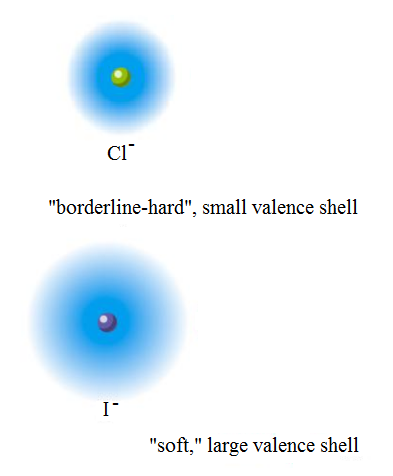
However,
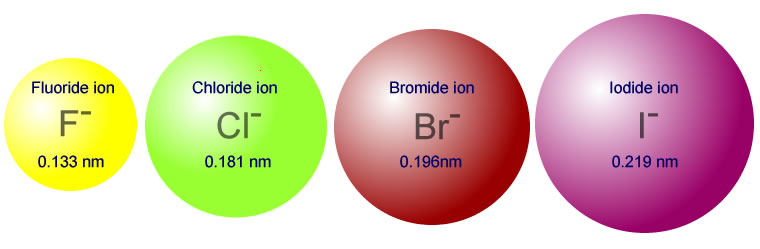
It's unclear how this all balances out without more context. So, keep in mind... context is extremely important, and defining your words is as well.

