Where is potential energy seen in chemistry?
1 Answer
The potential energy of a chemical bond is where one might see potential energy.
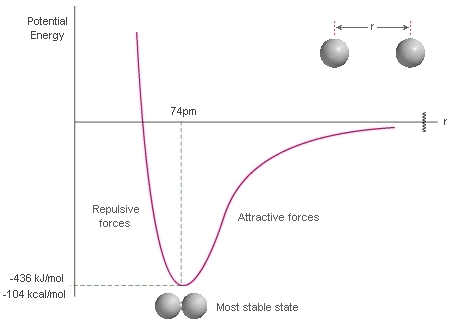
On the
-
As the two hydrogen atoms move closer together, the attraction between hydrogen A's valence electron and hydrogen B's proton increases. Same with hydrogen A's proton and hydrogen A's valence electron.
-
An energy of attraction is conventionally negative, so we move downwards on the potential energy curve.
If we move leftwards past the
-
When the two hydrogen atoms are too close, each proton in each nucleus repels the other. This is a nuclear repulsion energy.
-
This energy of repulsion is conventionally positive, so we move upwards on the potential energy curve.
A molecule wants to be as stable as possible; if it is unstable, it breaks apart.
So, the molecule prefers to be at the
#-"436 kJ/mol"#
This potential energy is the energy leftover, stored in the bond, after making the
If the bond breaks, the potential energy is absorbed back into the atoms as kinetic energy as the atoms move far apart.

