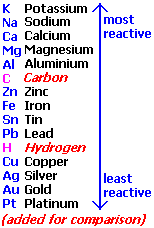
Will magnesium react with sodium sulfate?
1 Answer
Jun 7, 2016
No reaction will occur because Mg is less reactive than Na.
Explanation:
A single-replacement reaction would need to occur for
With your question this is not the case for sodium, as it is higher than magnesium and so it would rather NOT be reduced; thus, nothing will take effect.