Question #f4b81
1 Answer
Seven electrons.
Explanation:
Your starting point here will the electron configuration of a neutral iron atom,
Iron is located in period 4, group 8 of the periodic table and has an atomic number equal to
#"Fe: " 1s^2 2s^2 2p^6 3s^2 3p^6 3d^6 4s^2#
Now, a set of four quantum numbers is assigned to each of those
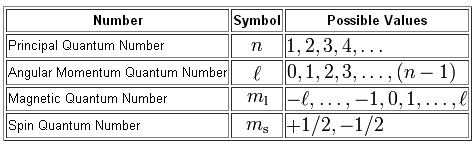
Your goal here is to figure out how many electrons have
#n + l + m_l = 4#
It's important to realize that for every set of
Starting with the electrons located on the first energy shell,
#n= 1" " implies" " l = 0 " "implies " "m_l = 0#
The two electrons located on the first energy level can have only one set of
For the electrons located on the second energy level,
#n=2 " "implies" "{ (l=0 " "implies" "m_l = color(white)(-1) 0 color(white)( +1)), (l=1 " "implies" " m_l = {-1, 0, 1}) :}#
This time you can have
#n = 2, " "l = 1, " "m_l = +1 " "implies" " 2 + 1 + 1 = 4#
This set of
For the electrons located on the third energy level,
#n=3 " "implies" "{ (l=0 " "implies" "m_l = color(white)(-1) 0 color(white)( +1)), (l=1 " "implies" " m_l = {-1, 0, 1}), (l=2 " "implies" " m_l = {-2, -1, 0, +1, +2}) :}#
This time you can have
#n = 3, " " l=1, " "m_l = 0 " "implies" " 3 + 1 + 0 = 4#
This set of
Notice that you can also have
#n=3, " " l=2, " " m_l = -1 " " implies" " 3 + 2 + (-1) = 4#
This set of
This is the case because iron has a total of
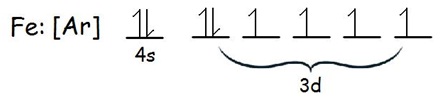
If you assign
Finally, for the electrons located on the fourth energy level,
#n=4 " "implies" "l=0 " "implies" "m_l = 0#
Notice that iron has only two electrons on its fourth energy level, both in the
These two electrons share the
#n=4, " " l=0, " " m_l = 0 " "implies" " 4 + 0 + 0 = 4#
So, the total number of electrons for which
#n + l + m_l = 0#
is equal to
#"no. of e"^(-) = overbrace(color(white)(a)2color(white)(a))^(color(blue)("located in 2p"_ y)) + overbrace(color(white)(a)2color(white)(a))^(color(purple)("located in 3p"_ z)) + overbrace(color(white)(a)1color(white)(a))^(color(brown)("located in 3d"_ (yz))) + overbrace(color(white)(a)2color(white)(a))^(color(red)("located in 4s")) = color(green)(|bar(ul(color(white)(a/a)color(black)("7 e"^(-))color(white)(a/a)|)))#

