Question #2bcc3
1 Answer
Here's my understanding of the difference.
Explanation:
To obtain any of these geometries, you must first draw the Lewis structure for the molecule.
Let's say you are dealing with water,
You draw the Lewis structure:

Then you use VSEPR theory to predict the electron pair geometry.
The electron pair geometry is the arrangement that the electron pairs will assume.
You see two lone pairs and two bonding pairs (4 electron pairs).
VSEPR tells you that the electron pairs will arrange themselves at the corners of a tetrahedron, with angles of about 109.5°.

The actual
Nevertheless, we say that the electron pair geometry is tetrahedral.
Our measuring instruments can't "see" the lone pairs. They can "see" only the bonds.
Hence, the molecular geometry is the arrangement of just the bonds (ignoring the lone pairs).
Since the H-O-H bonds are at an angle of 104.4°, we say that the molecular geometry of water is bent.
Here's a table of some common electron pair and molecular geometries.
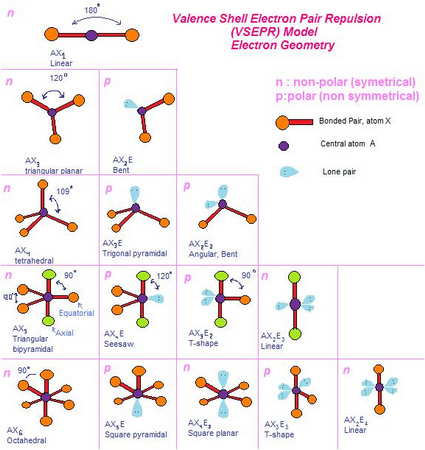
(from rinioktavia19942.wordpress.com)
The names of the electron pair geometries are in the left hand column, and all the names represent the molecular geometries.
Here's an interesting video on VSEPR theory.

