Question #e9fc3
1 Answer
See explanation.
Explanation:
Chelation is simply a type of bonding that requires the formation of dative covalent bonds, which you'll often see referred to as coordinate bonds, between one or more multidentate ligands and a metal cation.
The thing that stands out here is the fact that one or more ligands must be multidentate, meaning that they must form at least
As you know, a dative covalent bond is very similar to a classic covalent bond. However, the thing to remember here is that when two atoms form a dative covalent bond, both bonding electrons are supplied by one of the two atoms.
Simply put, a dative covalent bond is formed when one atom donates a pair of electrons to bond with another atom.
Now, chelation takes place when you have a metal cation and at least one multidentate ligand called chelating agent.
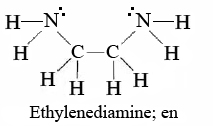
A classic chelating agent to use as an example would be ethylenediamine (en),
Each molecule of en can form
So, for example, you can have cobalt(III),
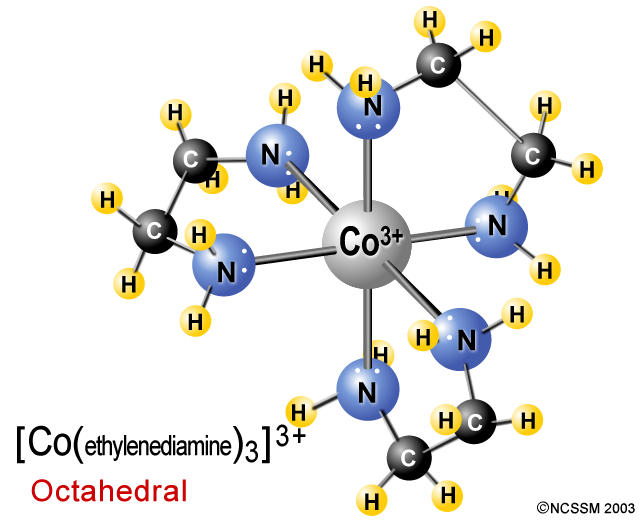
This complex ion is called tris(ethylenediamine)cobalt(III). Notice that each en molecule forms
You can also have something like this
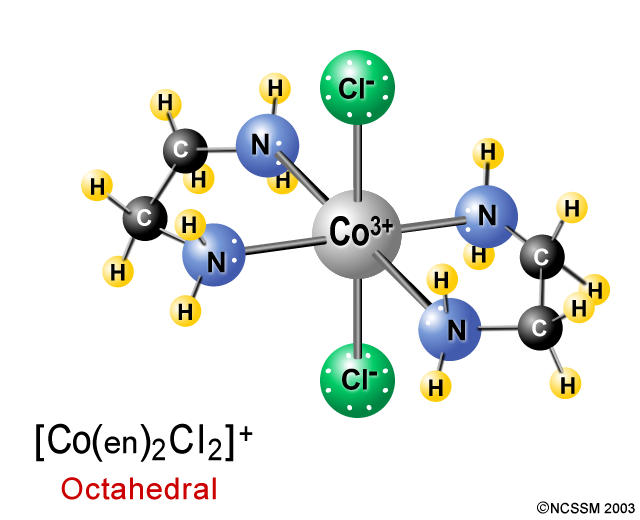
In this case, one of the three en chelates has been replaced with two chloride anions,
The resulting complex ion is called trans-dichloro-bis(ethylenediamine)cobalt(III). You can also have the cis isomer, cis-dichlorobis(ethylenediamine)cobalt(III), which looks like this
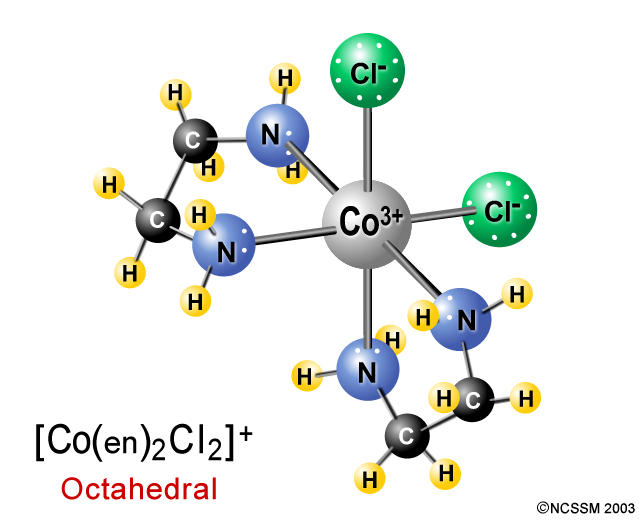
So, to sum this up, chelation is a process that involves the formation of dative covalent bonds between at least one multidentate ligand and a metal cation.