Question #db592
1 Answer
Here's what I got.
Explanation:
Your starting point here is the electron configuration of a neutral atom of iron,
#"Fe: " ["Ar"] 3d^6 4s^2#
As you know, when an atom of iron forms a
It is very important to keep in mind that these two electrons will come from the
The iron(II) cation,
#"Fe"^(2+): ["Ar"] 3d^6#
So, you know that the two electrons that are being lost when iron forms the iron(II) cation are coming from the
A total of four quantum numbers can be used to describe the location and spin of an electron in an atom.
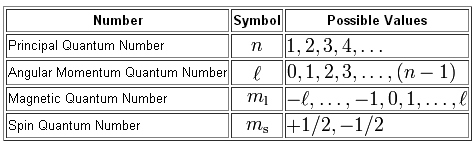
The principal quantum number,
#n = 4 -># the fourth energy level
The angular momentum quantum number,
#l=0 -># the s-subshell#l=1 -># the p-subshell#l=2 -># the d-subshell#l=3 -># the f-subshell
Your electrons are located in the
#l = 0 -># the s-subshell
The magnetic quantum number,
The s-subshell can only hold one orbital, since
#l= 0" "# and#" "m_l = -l, ..., -1, 0, 1, ..., +l#
The two electrons will thus have
#m_l = 0 -># the s-orbital
Finally, the spin quantum number,
#m_s = -1/2 -># an electron having spin-down#m_s = +1/2 -># an electron having spin-up
In your case, both electrons are coming from the same orbital, which means that the ymust have opposite spins. You can thus say that the two electrons can be described by the following quantum sets
#color(green)(|bar(ul(color(white)(a/a)color(black)(n = 4, l=0, m_l = 0, m_s = -1/2)color(white)(a/a)|)))# An electron located on the fourth energy level, in the
#4s# subshell, in the#4s# orbital, having spin-down
#color(green)(|bar(ul(color(white)(a/a)color(black)(n = 4, l=0, m_l = 0, m_s = +1/2)color(white)(a/a)|)))# An electron located on the fourth energy level, in the
#4s# subshell, in the#4s# orbital, having spin-up

