Question #2a9e4
1 Answer
Aug 31, 2016
THE REACTION IS CALLED ENDOTHERMIC REACTION
Explanation:
https://socratic.org/s/axvZR3EF
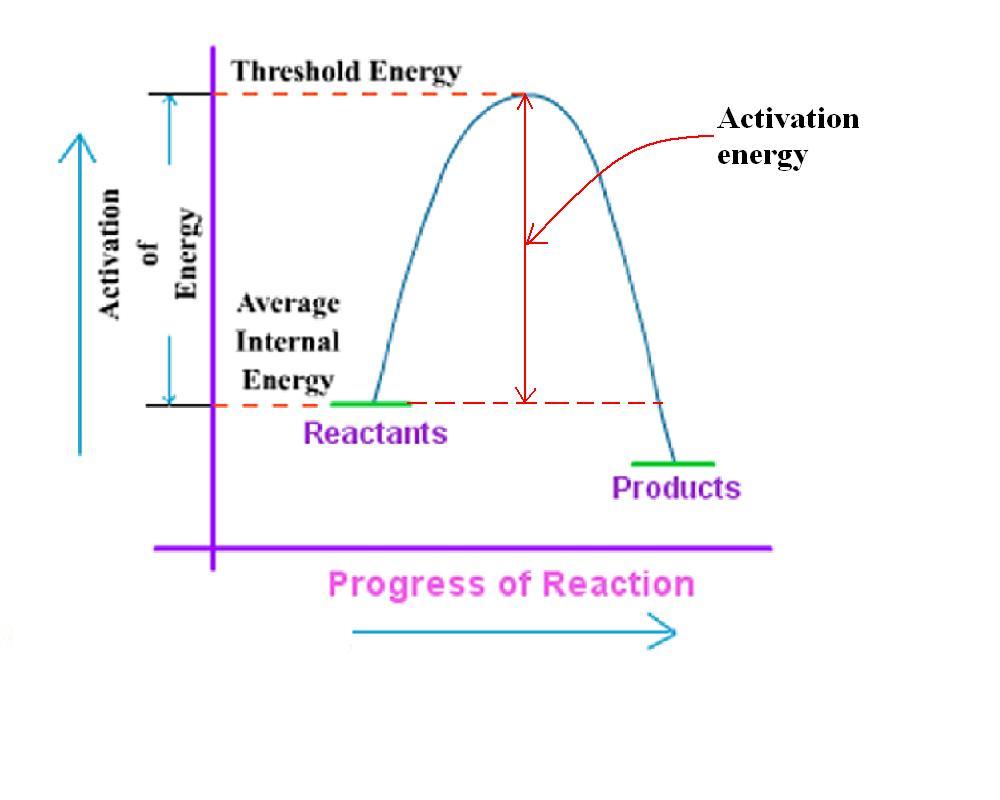
This figure is obtained from the link given above.In this link concept of activation energy has been explained in detail.
Let
So
Now
Again given cindition is

